Did my EcoRI restriction digestion work? picture included! - (Jun/27/2012 )
Good day to all,
In response to Papaver, the clones I chose are 7, 8, 9 , and 10. I actually avoided 6 and 12 because they were too bright at first, but today I decided to include them in my EcoRI digestion that cuts at the vector around the insert. What do you mean by negative control please?
In response to Phage 434, NdeI and XhoI are meant to cut at my insert.
Today, I did as you two wished I used another enzyme and the results seem a bit more promising.
Here is an image, ![]()
Sorry the image is not that clear.
The first two bands on the left hand side, particularly the second one from left seem to have the correct construct of interest at size 585bp.
So should I preform NdeI and XhoI digestion on these two and see the result?
Thanks!!
Hi Fluffy,
negative control means you are adding water instead of template to the reaction. If a PCR band occurs you know that there is a condamination. And, often those bands are not that bright as the real positive ones...
So, if you are doing a EcoRI digestion with some other clones, wait for the result and then chose the clone that has the right size, either one of the Eco-digestion or No. two of the picture you have posted here.
I'd recommend you read the NdeI FAQ. Note what it says about DNA contaminants, and also the comment on long digestiosn. It is a good practice to get into the habit of reading these FAQs when using a new enzyme. Or to use a protocol that never requires you to use new enzymes.
https://www.neb.com/nebecomm/products/faqproductR0111.asp#2089
Thanks Papaver and Phage 434, these are good points to keep in mind. I will consider them as I continue my work ![]() . I will keep you updated!
. I will keep you updated!
Hi I am back ![]()
I digested one of my clones with NdeI and XhoI and YAY!! it worked..I cut the band for the purpose of gene clean, and here is a picture of my insert after digeston and gene clean 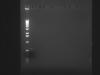
Now my next step is ligation. The concentration of my insert is low after gene clean 1.5-2ng/uL. I am worried it won't be sufficient for ligation.
My double digested pET14b vector is 23.3ng/uL.
Should I redigest my insert or is this concentration good enough for ligation?
Papaver I am not sure if you recall, we agreed on a ligation protocol for my other insert on this same thread and it worked well. Do you think I should use it again for this one, even though my insert concentration is lower.
Any ideas would help!!
In the words of Trof, anything measured by nanodrop under 10 ng/uL is a number randomly generated. I would say it is too little to trust what you have there.
Hi,
so it can work with such low amounts,I did it once with ~ 1 ng/µl. Usually I work with amounts of 4 - 15 ng/µl and it always works fine. Just do the same protocol as before, maybe with less vector concentration (20-30 is thoroughly enough)...and using therefore more in the transformation mix.
But in parallel I would repeat the digestion using more template and set up two or three reactions at the same time if you have enough template...otherwise prep new vector. Then you would have enough for ligation.
Hello,
Thanks for the replies, ascacioc and Papaver.
In regards to making a new insert digestion with template sounds like an idea to me and will do that in parallel to my other work.
On the other hand, Papaver, you mentioned I can use the same protocol that we agreed on earlier which was
DD pET14b vector 2.5uL
DD insert 5.5uL
5X ligase buffer 1.5uL
T4 DNA ligase 0.5uL
I didn't understand what you mean by less vector concentration? So I shouldn't use 2.5uL? Do you mean I use less, wouldn't that mean I would have to add water to make up 10uL
Also last time I added 2uL of my ligation for transformation mix, do you suggest using more this time?
Thanks again
HI Fluffy,
by using less I meant the total amount of DNA. You always have to adjust your protocol to your DNA concentrations.
Helpful therefore is: use vector concentrations of 20-50 ng; a molar ratio of insert: vector = 4:1; final buffer concentrations has to be 1x, use 0.5 - 1 µl ligase.
The final volume of you ligation mix does not have to be 10 µl. You can vary it in a way you need it. You may also read the recommendations of the company you got the ligase from.
So this time it would be better to use less vector DNA because you will need more insert DNA.
Try this: 2 µl vector (~ 40 ng)
11 µl insert (~ 16.5-22 ng)
1.5 µl 10xbuffer (= 1x)
0.5 µl ligase
If you still have only 5x ligase buffer, then: 4 µl of 5x buffer, 2.5 µl of vector, 13 µl insert and 0.5 µl ligase.
You also can vary the volume for your transformation. If you want to make sure you get some colonies, do two or three transformation at the same time using, let's say 1 µl, 2µl and 3µl of DNA. When I had difficulties with my cloning (usually when I had to use some old plasmids) I used to transform up to 10 µl (for 200 µl competent cells). When I remember right, the volume of your competent cells was 50 µl. So do not add more than 5 µl (which would be 10 % of the total volume).
Thanks Papaver. Had a vacation. Did those ligations today and hope for the best!
