 |
Top : Cell Biology : Cell Culture : General Procedures : Maintenance of Cell Line : Protocol for Caco-2 cell culture
Protocol for Caco-2 cell culture | |
| Author: Arun Malik and Hariom Yadav* | |
| Affiliation: Nutritional Biotechnology Department, National Agri-Food Biotechnology Institute, Mohali, India | |
| Source: Protocol Online | |
| Date Added: Tue Jan 22 2013 | |
| Date Modified: Tue Jan 22 2013 | |
| Abstract: This protocol describes about how Caco2 cells should be maintained into the labs and common cares which should be taken during culture process. | |
Introduction
Cell culture is very important approach to investigate various molecular
mechanism(s) and phenotypes in cells that are distinct or separated from whole
animal system. Caco-2 cells are continuous lines of heterogenous human
epithelial colorectal adenocarcinoma cells. These cells are derived from human
large intestine and become differentiated and polarized like small intestinal
cells, when grown in specific cell culture conditions. Caco-2 cells from
American Type Culture Collection (ATCC) grown in basic culture medium in tissue
culture flasks/discs. Normally cultures kept at 37°C
in a 5% CO2/95% air atmosphere. Caco-2 cells are mostly used as a
confluent monolayer on a cell culture inserts filters. The Caco-2 monolayer is
widely used across the pharmaceutical industry as an in vitro model of human
small intestinal mucosa to predict the absorption of orally administered drugs.
We are discussing a protocol which can be normally adopted to culture and
maintain these cell lines in the laboratories.
Materials and reagents
- Standard cell culture facility
- Laminar hood
- Humidified CO2 incubator
- Inverted microscope
- Tissue culture flasks
- Serological pipets
- 50 ml centrifuge tubes
- Dulbecco’s Modeified Eagle Medium (DMEM)
- Fetal Bovine Serum (FBS)
- Penicillin-streptomycin (P/S) solution
- Phosphate Buffer Saline (PBS) without calcium and magnesium
- Trypsin-EDTA solution
- Caco-2 cells (ATCC Catalogue No.-HTB-37)
Procedure
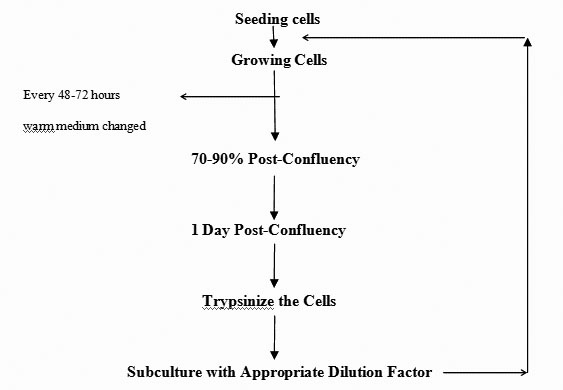
- Cacao-2 cells cultures routinely maintained in tissue culture flasks/ discs in Dulbecco’s Modified Eagle Medium (DMEM) medium containing 10% Fetal Bovine Serum (FBS) and 1% penicillin-streptomycin.
- Cells split into 2.5x10 cm flasks/discs every 3-4 days with re-feeding of every 48-72 hours.
- Remove culture medium from flasks/discs and wash monolayer with 1x Phosphate Buffer Saline (PBS).
- Add 2-5 ml of trypsin solution; incubate at 37°C for 1-4 minutes to remove the cells from surface.
- Add 15 ml of DMEM supplemented with 10% FBS and 1% P/S media to inactivate trypsin and thoroughly pipette up and down to break the cell clumps.
- Transfer required volume of warm media to seed cells in a sterile flask/disc.
- If required count the cells using standard cell counting procedure (described somewhere else).
- Excess cells may be re-seeded in to a new flask. Every time cells are passaged and the passage number increased by 1.
- The media usually changed every 48-72 hours by aspirating off old media
and replace it with new media.
Helpful-Hints
- Handle the solutions and other amenities with care and always bear gloves in your hands.
- Use of microbial safety cabinet / air-hood is most important piece of the cell culture process. During operation, it will provide a clean working environment for the procedure and avoids any kind of contamination.
- Check the incubator for set-up to provide CO2 at required level (5%). Always check temperature of incubator regularly to ensure the levels are being maintained correctly.
- Keep monitoring for gas levels in CO2 cylinders.
- Take care for using sterilize procedures/habits as much as possible, to avoid contamination.
- Always warm the media before using with cells.
Notes:
*To whom corresponding should be made: Dr. Hariom Yadav, Ramalingaswami Fellow, National Agri-Food Biotechnology Institute, Mohali, India. Email: yadavhariom@gmail.com