Transfer buffer for tris tricine gel - (Jun/04/2015 )
i recognized the blue smear as tracking dye. but i also looked at its appearance. the pinching near (or at) the buffer front is a strong clue that there is something affecting migration (eg salt, detergent, etc).
although 2:1 buffer:sample is acceptable, have you tried 1:1?
one of the problems may be that your loaded volume may be too high to ensure compact banding of the lower weight proteins. this may be caused by actual sample volume or by gel loading method.
also, it is a good practice to load the same volume of all samples and standards just dilute everything with the same diluent to equal final volumes. this will minimize effects caused by buffer concentration differences.
I did tris tricine gel. Separation looks just fine. I wonder why does it get so darkened when i do imaging? I am using 0.2 micron pvdf instead of 0.45. Because it gets darkened at early exposure i cannot extend exposure time. For other larger protein, it runs just fine. What extra care we need for small sized pvdf? Please look at the pic attached. In the marker lane i have used pencil marking to show ladder. It came as white after chemiluminiscence.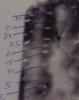
High background is often caused by dirty blocking solution (e.g. bacterial contamination) or too much secondary (too high a concentration or too long an incubation).
