Transfer buffer for tris tricine gel - (Jun/04/2015 )
Hello everyone
This is my first post.
I need help regarding the selection of transfer buffer for tris tricine gel.
I m looking for a band in 6 kb. I m following the protocol by Herman schagger 2006 nature about tris tricine page. The band runs perfect with nice separation. But when i image it the membrane is dark like a ghost. I tried several buffer condition for transfer of my low mw protein. The standard towbin buffer without sds. 25 mM tris base, 192 mM glycin, 20 % methanol. At one time i adjusted the ph to 8.3 and at other time i did without adjustment n it was at 8.42. Both the time the membrane was dark
I use pvdf membrane. Dip in 100 % methanol for 30 sec n in transfer buffer for 15 mins before transfer. We have trans blot semidry transfer aparatus from biorad. Transfer at 13 v for 15 mins. I never allow my membrane to dry out at any time. Ponceau stain is showing protein at low mw area. Gel stain shows efficient transfer at low mw area. But when i do imaging, ECL imaging, after 30 sec exposure, everything gets too darkened. Markers appear as white in dark background . Could not figure out where the problem is. Oh i do block overnight in 5 % milk . Please help
If you can see stained bands on your membrane by ponceau, it would indicate that the problem is not transfer, but rather to do with your antibody detection.
I think the problem is bacterial or yeast contamination of your milk, this will often result in non-specific binding of the secondary (maybe the primary too) to the bacterial proteins on the membrane. The contamination could be a contamination of either the milk powder or the buffer. Best to throw both out and get new ones. Overnight blocking is excessive, and shouldn't be necessary.
I see a smeared bands on ponceau staining. I could not see any bands. The gels are clear at lower end. I block overnight at 4c. Its a flag antibody and its working just fine with my other protein. I dont think its a bacterial contamination because same protocol is giving me good bands with other larger protein. Is 6 kb too small for a 1 copy of flag antibody to detect?
have you stained a parallel gel to see the migration of the protein?
do you use a different primary antibody than you use for the larger proteins?
your primary may be reacting with the milk. try a different block (bsa, normal serum from the species of the secondary,...). also, add block to the antibody solutions, if you aren't already doing it, to preabsorb any antibodies which will bind to the blocking agent.
I m posting you the image of my gel and membrane after transfer
My image got inverted here. The top pink band is 2 kb
N here is the picture of my gel after transfer after Comassie staining.
from the looks of the post-transfer gel, i think you may have had contact problems. that would allow the bands to smear as they crossed over to the membrane.
(for some reason, i can't open the pics of the membranes, so can't comment on them)
can you show the results from a processed blot?
Actually the gel picture was taken with Kim wipes back ground and it got crumbled. Here's the better image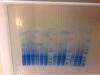
much better, and now i can see the blot, too.
what i'm seeing is either a huge overload of tracking dye or, more likely, components in your sample or sample's buffer (before adding the sds buffer) are causing disruption of the low mw portion of the gel.
this can be caused by detergents, lipids, salts, etc.
there may also be some contact (bubble) problems.
The blue smear you are seeing in the pvdf membrane is dye front. You can see that in gel too. Since I am looking the band at 6 kb. It escapes that smeared area, so i was not bothered. Tris tricine gels are so painful to run they are not as easy as tris glycine. Plus the dye front does not go away like in tris glycine. I religiously followed Herman's protocol. But in that paper he uses different transfer system and there is no recipe for buffer too. We have trans blot sd semidry transfer cell from biorad for transfer thing so i always think that i am doing my transfer wrong. The markers are transferred so well in the membrane but. The lowest one in pink is the 2 kd but i am afraid my proteins are not efficiently transferred.
My proteins concentration is quite high. Like 7microgram per microliter as measured by Bradford. I have had run different concentrations in that gel from 20 ng to 60 ng. I am using a commercial sample buffer tricine sample buffer biorad with 2 percent 2me at the time of gel run. I used like 2 parts sample buffer and 1 part protein sample.
So looking at the gel and membrane is it a transfer problem or the salts etc in protein sample? Shud i try dialysis?
