CO2 gradient/standard - (Mar/07/2014 )
Hi Protocol.
I am looking into measuring the CO2 release in some samples, I need the rate of the CO2 release over roughly 2-4 hours, I am going to use a pH indicator but I need some kind of standard chemical/reaction to produce the standard curve. I havent really been able to find anything usefull, so was hoping that one of you guys had any recommendation for measuring a known/controleld CO2 release over some hours? Preferably 4 hours, but less can do.
Best regards
Jonas
The classic way to do this would be to use a radioisotope pulsed into the samples and then measure output over time. Depending on where it is released (liquid? gas phase?), you can measure this with a mass-spec of some sort. Gas would be best measured using GC-MS, and I guess liquid could be done with LC-MS, but I'm no expert. This would obviate the use of a standard reaction to compare release to, but would be a definitive answer and much much more accurate than a pH change measurement.
boy, does bob1's response bring back memories.
way back when...
we used to capture 14co2 in a sealed vessel with koh or naoh in a center well and count. with a septum, you could withdraw aliquots at various time points.
mdfenko on Mon Mar 10 12:00:16 2014 said:
boy, does bob1's response bring back memories.
way back when...
we used to capture 14co2 in a sealed vessel with koh or naoh in a center well and count. with a septum, you could withdraw aliquots at various time points.
Do you have any information/protocol in regard to such a setup?
this is a graphic similar to what we used. the flask was 25ml with or without a sidearm (D). the septum (A) was rubber with the center well suspended as shown (B) and filled with 2M naoh or koh. the reaction was started by addition of the labeled substrate either through the sidearm (with a rubber septum sealing the arm) or through the main septum (avoiding the center well).

mdfenko on Mon Mar 10 13:44:22 2014 said:
this is a graphic similar to what we used. the flask was 25ml with or without a sidearm (D). the septum (A) was rubber with the center well suspended as shown (
and filled with 2M naoh or koh. the reaction was started by addition of the labeled substrate either through the sidearm (with a rubber septum sealing the arm) or through the main septum (avoiding the center well).
So what you would do is put the syringe through A and take the sample from the well in B? And is the center well a custome made solution or can they be bought, havent seen such as setup before. Sorry for all the questions. Ah sorry, my google skills arent to good on this monday I found it. Unfortunately I think it would be to much work since I was trying to do my setup in well plates.
yes, we would take the sample from the well by syringe through the septum to the well.
i found the product information for 10ml incubation flasks and accessories (is this what you found?)
Wouldn't be the classical Warburg apparatus the type of instrument you need? Actually I guess most or all are thrown away already, since it's pretty old, anyway perhaps some labs with physiological focus still use them or stored them (I once had a practical course using it, but long ago and forgot most...).
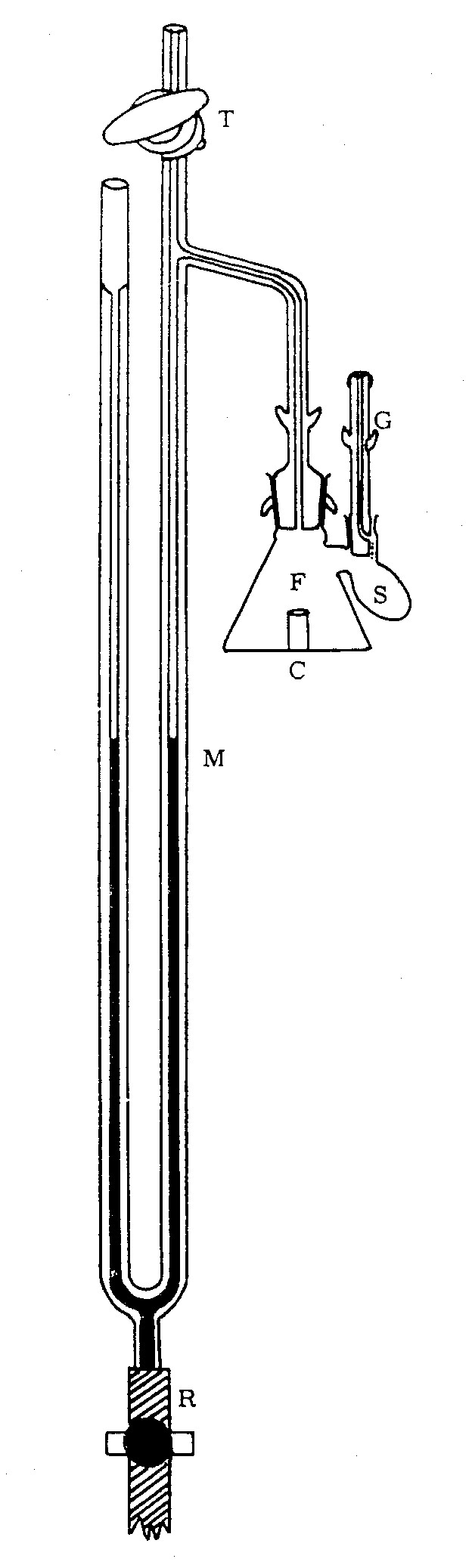
have a look how it works:
https://www.youtube.com/watch?v=M-HYbZwN43o
nope, wasn't the warburg. check the link in my last post for the apparatus that we used.
well he said he needs the rate over a few hours and my guess was then release rate over time, and not only at the endpoint only. Anyway the flasks are surely easier to use and still purchasable, Warburg will be difficult ![]() .
.
