Yet another contamination question - SF9 cells (Feb/28/2011 )
Hi. I've been working with SF9 cells for almost 2 years now and never had any sort of problems. Recently our lab technician neglected changing the Virkon soup in the waste disposal flask and as such, we got green sporulating mold growing in there over the weekend. Obviously the flask was removed carefully and the lab cleaned thoroughly afterwards, with Trigene and Ethanol (except for the floor).
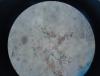
The next day, when checking my cells (which we grow in spinner flasks with a loose cap) I noticed 2 things: 1. when coloring the cells with trypan blue some of the preps had web-like structures clumping cells and there was a lot of junk around. I emphasise "some" because other times when doing a trypan blue coloration on the same line, the same day, these wouldn't appear. 2. even without coloration I started noticing long filaments. Not many (5-6 for both chambers of the haemocytometer).
I've attached pictures for both cases.
We then decided to bin everything just in case (cells, media, trypan) and restarted a new cell line. However I see the same thing now as before (occasional, isolated filaments). A professor in our lab suggested that they might be cellulose filaments resulting from wiping the haemocytometer with white tissue paper, but I am skeptical. Now I don't know if it's paranoia and if these things were always there (you know how when routine sets in, you tend to blank certain recurring things) or if it's indeed contamination, so I need your suggestions.
Other, maybe relevant, observations:
> The presence of these things doesn't seem to influence cell viability, just cell growth. Cell growth has slowed down considerably and they hover around 1x106 for long periods (days).
> When using trypan blue the filaments stain. I've read about fungi and it seems that for most species the outer wall is permeable to trypan blue.
> The number of filaments doesn't seem to increase at a high rate, if at all. Nothing else indicates contamination (culture smells as usual, nothing visible floating around, even after 5-6 days).
> I'm using pen-strep, but not fungizone.
> When looking at trypan blue alone, I could see a lot of junk and debris, probably because it's not filtered, so it is possible that the web-like thing around the cells could be due to filthy trypan (we're in the process of recieving new dye soon, which we will filter). Also when looking at just the surface of the haemocytometer after ethanol-wiping it, I can sometimes see 1-2 filaments so the cellulose from tissue theory might be correct.
Are you guys seeing any of this? And if not, does it look like fungus? Thank you for your help.
My thoughts:
1. doesn't look like any kind of fungal contamination I've ever seen or experienced
2. can you only see it in the trypan preps? Or in your flasks too. 5-6 filaments over two chambers seems a lot to me, so I would expect to see them in the flask too, if they were in fact contamination
3. I tend to agree with your prof about it being from tissue
Hola, when you have a fungi infection, you coud see hiphae as cotton fibers that spread by the culture quickly, moreover the culture shows a different turbidity and you could see under microscope hiphae and lot of diminute points between cells. (As you can see I´m a bit filthy, because sometimes I have an infection specially in the wells of the edge of plates incubated long time). Other source of materials in suspension is the serum. After inactivation with the freezing of aliquots some proteins precipite (as fibers) and appears in the ulterior tawing.Only I¨M not able to explain the slow grothw. Buena suerte
The other possibility is mycoplasma. You probably should send out a sample for identification just to be sure.
leelee on Tue Mar 1 03:15:48 2011 said:
My thoughts:
1. doesn't look like any kind of fungal contamination I've ever seen or experienced
2. can you only see it in the trypan preps? Or in your flasks too. 5-6 filaments over two chambers seems a lot to me, so I would expect to see them in the flask too, if they were in fact contamination
3. I tend to agree with your prof about it being from tissue
I can't give you any answers as to what it is, but I can say that I have seen something very similar when preforming cell counts, however, when I see these fibers it's never been more than 1 or 2 on the entire hemocytometer.
These are filaments from the tissue paper. They should stain bright blue with trypan blue. Try using low residue wipes (e.g. kimwipes) to prevent this issue.
Edited to add:
Definitely not mycoplasma! You would never see this sort of structure or of that size with mycoplasma.
Did you ever get to the bottom of this issue? We are seeing the exact same thing. Any thoughts on how to get rid of this or fix this issue? Thanks!!
The pictures supplied show filaments from tissue paper and possibly some precipitates from FBS, these are not fungal hyphae.
Slowdown of culture growth could be a number of different things, including old medium, change of batch of serum, mycoplasma contamination, or Hayflick limit.
I agree that the filamentous structures and crystal looking structures look likely to be tissue bits, but the bright circles of similar size look like yeast to me. If it is yeast, 0.2uM filtration would work. You could also DAPI stain to look for nucleii or DNA (for larger bacteria). Definitely NOT mycoplasma. They are much much smaller, and most can't survive long outside of the cell. I don't know if all yeast will cause yellowing of medium, but some are very slow growing, so you might not see the overnight cloudiness you see with faster growing bacteria like E. coli or S. aureus / S. epidermidis.
hi. I've got the same problem recently while I was staining my Huh-7 cells with trypan blue .
the cells are perfect with out the stain and also the flask doesn't show any sign of contamination but when I stain the cells, the same particles as yours appear on Hemocytometer.
I don't know if this is a fungal contamination, just artifact or paper tissue residues.
I am wondering if you could find out what these particles are and the solution of removing them.
I appreciate you and anyone who can help me through this cell culture problem.
I attach the photo of the cells here.