 |
Top : Immunology : ELISA : ELISPOT : ELISPOT
ELISPOT | |
| Author: Dr. Mohamed Tarek Shata | |
| Affiliation: Mohamed Tarek Shata, University of Cincinnati, Viral Immunology Lab | |
| Source: Protocol Online | |
| Date Added: Mon Feb 02 2009 | |
| Date Modified: Mon Feb 02 2009 | |
| Abstract: The usage of ELISPOT techniques to measure Cytokines (IFN-g, IL-4, or IL-10) | |
Human ELISPOT Techniques
|
|
|
| Typical images of human IFN-g ELISpot assay wells following development. Distinct spots are readily apparent in wells containing human PBMCs that were stimulated and cultured overnight with stimulus (left panel), whereas no spots are detected in wells containing non-stimulated cells (right panel). | |
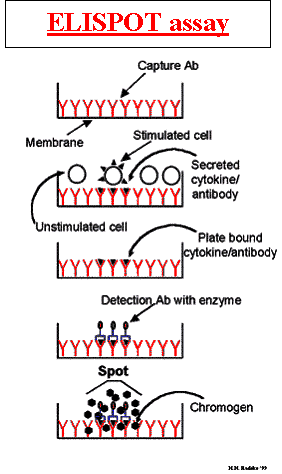
Principle of the assay:
The enzyme-linked immunospot (ELISpot) assay was originally developed for the detection of individual B cells secreting antigen-specific antibodies. This method has since been adapted for the detection of individual cells secreting specific cytokines or other antigens. ELISpot assays employ the quantitative sandwich enzyme-linked immunosorbant assay (ELISA) technique. A monoclonal antibody specific for a cytokine has been pre-coated onto a PVDF (polyvinylidene difluoride)-backed microplate. Appropriately stimulated cells are pipetted into the wells and the microplate is placed into a humidified 37°C CO2 incubator for a specified period of time. During this incubation period, the immobilized antibody in the immediate vicinity of the secreting cells binds secreted cytokine. After washing away cells and any unbound substances, a biotinylated polyclonal antibody specific for the cytokine is added to the wells. Following a wash to remove any unbound biotinylated antibody, alkaline-phosphatase conjugated to streptavidin is added. Unbound enzyme is subsequently removed by washing and a substrate solution (BCIP/NBT) is added. Blue-black colored precipitate forms at the sites of cytokine localization and appears as spots, with each individual spot representing an individual cytokine-secreting cell. The spots can be counted with automated ELISpot reader systems or manually, using a stereomicroscope.
A. IFN-g ELISPOT assays (MABTECH Cat No M34201-A) : According to manufacturer’s instructions:
- Coating of ELISPOT plates:
- Dilute the coating antibody (1-D1K) to 15 æg/ml in sterile filtered PBS, or bicarbonate buffer pH 9.6.
- Add 100 ml to each well of 96-well Millipore plate and incubate overnight at 4C.
- Wash the plate 4 times, and block the non-specific binding sites by adding 200 ml of blocking buffer (RPMI medium with 10% FCS).
- Isolation of mononuclear cells from peripheral blood
- Ficoll-hypaque PBLs, and isolated mononuclear lymphocytes.
- Wash the cells and count the viable cells.
- Resuspend the cells in tissue culture medium at RPMI with 10% FCS at a concentration 4X106 cells/ml. Add 100 æl of cell suspension/well.
- Stimulation and ELISPOT analysis:
- Adjust the stimulus concentration as 2X (for PHA use 1-10 æg/ml, for peptides use 1-10 æg/ml) and add 100 æl of the stimulus to the wells according to the protocol.
- Incubate the cells for 18 hr at 37C, in humid 5% CO2 incubator.
- ELISPOT development:
- Remove the cells by washing 6 times, 2 times in dist water and 4 times in washing buffer.
- Diluted the biotinylated mAb (7-B6-1 biotin) in Blocking buffer to 1 æg/ml. Add 100 æl to each well.
- Diluted the streptavidin alkaline phosphatase (1:1000) in Blocking buffer. Add 100 æl to each well.
- Incubate at room temperature for 1-2 hour.
- After washing add 100 æl of substrate, and incubate until dark spots emerge.
- Stop color development by washing in tap water and leave the plate to dry.
- Count the number of spots in a dissection microscope, or using automated machine.
B. IL-4 ELISPOT assays (MABTECH Cat No M34101-A): According to manufacturer’s instructions:
1. Coating of ELISPOT plates:
-
Dilute the coating antibody (IL-4-I) to 15 æg/ml in sterile filtered PBS, or bicarbonate buffer pH 9.6.
-
Add 100 ml to each well of 96-well Millipore plate and incubate overnight at 4C.
-
Wash the plate 4 times, and block the non-specific binding sites by adding 200 ml of blocking buffer (RPMI medium with 10% FCS).
2. Isolation of mononuclear cells from peripheral blood
-
Ficoll-hypaque PBLs, and isolated mononuclear lymphocytes
-
Wash the cells and count the viable cells.
-
Resuspend the cells in tissue culture medium at RPMI with 10% FCS at a concentration 4X106 cells/ml. Add 100 æl of cell suspension/well.
3. Stimulation and ELISPOT analysis:
-
Adjust the stimulus concentration as 2X (for PHA use 1-10 æg/ml, for peptides use 1-10 æg/ml) and add 100 æl of the stimulus to the wells according to the protocol.
-
Incubate the cells for 40 hr at 37C, in humid 5% CO2 incubator.
4. ELISPOT development:
-
Remove the cells by washing 6 times, 2 times in dist water and 4 times in washing buffer.
-
Diluted the biotinylated mAb (IL-4-II- biotin) in Blocking buffer to 1 æg/ml. Add 100 æl to each well.
-
Incubate at room temperature for 1-2 hour.
-
Diluted the streptavidin alkaline phosphatase (1:1000) in Blocking buffer. Add 100 æl to each well.
-
Incubate at room temperature for 1-2 hour.
-
After washing add 100 æl of substrate, and incubate until dark spots emerge.
-
Stop color development by washing in tap water and leave the plate to dry.
-
Count the number of spots in a dissection microscope, or using automated machine.
C. IL-10 ELISPOT assays (MABTECH Cat No M34301-A) : According to manufacturer’s instructions:
- Coating of ELISPOT plates:
- Dilute the coating antibody (9D7) to 15 æg/ml in sterile filtered PBS, or bicarbonate buffer pH 9.6.
- Add 100 ml to each well of 96-well Millipore plate and incubate overnight at 4C.
- Wash the plate 4 times, and block the non-specific binding sites by adding 200 ml of blocking buffer (RPMI medium with 10% FCS).
- Isolation of mononuclear cells from peripheral blood
- Ficoll-hypaque PBLs, and isolated mononuclear lymphocytes.
- Wash the cells and count the viable cells.
- Resuspend the cells in tissue culture medium at RPMI with 10% FCS at a concentration 4X106 cells/ml. Add 100 æl of cell suspension/well.
- Stimulation and ELISPOT analysis:
- Adjust the stimulus concentration as 2X (for PHA use 1-10 æg/ml, for peptides use 1-10 æg/ml) and add 100 æml of the stimulus to the wells according to the protocol.
- Incubate the cells for 40 hr at 37C, in humid 5% CO2 incubator.
- ELISPOT development:
- Remove the cells by washing 6 times, 2 times in dist water and 4 times in washing buffer.
- Diluted the biotinylated mAb (12G8- biotin) in Blocking buffer to 1 æg/ml. Add 100 æl to each well.
- Incubate at room temperature for 1-2 hour.
- Diluted the streptavidin alkaline phosphatase (1:1000) in Blocking buffer. Add 100 æl to each well.
- Incubate at room temperature for 1-2 hour.
- After washing add 100 æl of substrate, and incubate until dark spots emerge.
- Stop color development by washing in tap water and leave the plate to dry.
- Count the number of spots in a dissection microscope, or using automated machine.
References for ELISPOT assays
Czerkinsky, C., Nilsson, L.angstrom., Nygren, H., Ouchterlony, O., and Tarkowski, A. 1983. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 65:109-121.
Miyahira, Y., K. Murata, D. Rodriguez, et al., Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J. Immunol. Meth., 1995. 181(1): p. 45-54.
Lalvani, A., R. Brookes, S. Hambleton, W.J. Britton, A.V. Hill, and A.J. McMichael, Rapid effector function in CD8+ memory T cells. J Exp Med, 1997. 186(6): p. 859-65.
Lalvani, A., R. Brookes, R.J. Wilkinson, et al., Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci U S A, 1998. 95(1): p. 270-5.
Asai, T., W.J. Storkus, and T.L. Whiteside, Evaluation of the modified ELISPOT assay for gamma interferon production in cancer patients receiving antitumor vaccines. Clin Diagn Lab Immunol, 2000. 7(2): p. 145-54.

