PCR product stays on agarose gel well !! - I need help, what is it happening? (Jun/30/2009 )
Hello,
Has anyone else encountered this problem: when I run my latest PCR product, most of the DNA seems to stay in the well, I can see the ethydium bromide signal coating the well, does not move at all.
I get bands at expected bp sizes with different primers sets, but the strongest signal of all is the stuff still inside the well.
I already tested equivalent amount of template by itself (no PCR reaction) and it does run down the gel, does not get stuck in the well.
I also ran all the individual components of the reaction (buffer, water, MgCl, Taq, dNTPs, or primers, no reaction) and none show anything in the well.
I also ran a PCR reaction without template and did not show any signal in the gel.
Could there be a chemical contaminant that gets "activated" by the PCR reaction temperatures and binds DNA into a big blob that cannot move down the gel? This is my favorite hypothesis.
Is it possible to have a HUGE amplicon? Any other ideas? Many thanks
See attached photo. All lanes have 1.4 uL buffer and water to 14 uL total. Lanes as follows:
1) 100 bp ladder
2) 250 ng genomic DNA template
3) water
4) PCR product using 250 ng same DNA as (2)
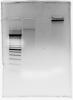
If the template was genomic DNA, I suppose this could be a big blob of template because of random annealing...? Was your template-only control subjected to temperature cycling?
I agree with homebrew...looks like gDNA which did not amlify.
Did your primers work with this template earlier? And do you get the same result when using another primer pair?
And another "theory" have you tried to clean up your PCR product (column or precipitation, not enzymatic), maybe something in your PCR buffer causes "strange" running of your template? Sounds weired, but we were using an "enhancer" mix for our soil DNA extracts which resulted in PCR products running in the wrong direction. When we column (or PEG) purified our samples they suddenly were running correct again.
The template-only control did not go thru temperature cycling.
HomeBrew on Jun 30 2009, 09:50 PM said:

One of my primer sets (I was testing several) seem to be working but I still need to optimize to minimize other bands. In this gel I just ran a random PCR product, probably one of the primer sets that did not work.
I do get the correct band when using primers for a different gene amplicon that we use in another project and I was using here as positive control. But I also get the big blob in the well.
My boss thinks the big blob in the well may be using up my primers, maybe even the dNTPs, if it is some king of amplicon, so i could get a better specific band if I could get rid of this non-running blob.
This gDNA was cleaned with the QIAquick PCR purification kit BEFORE running the PCR reaction. Maybe I should clean it also AFTER the PCR reaction?
Anyway, we had a student run a PCR will run gel today, with a plate rather than tubes like I was using, and some of my tubes also. Will know soon if there is something wrong with the tubes, or with me ![]()
gebirgsziege on Jul 1 2009, 02:51 AM said:
Did your primers work with this template earlier? And do you get the same result when using another primer pair?
And another "theory" have you tried to clean up your PCR product (column or precipitation, not enzymatic), maybe something in your PCR buffer causes "strange" running of your template? Sounds weired, but we were using an "enhancer" mix for our soil DNA extracts which resulted in PCR products running in the wrong direction. When we column (or PEG) purified our samples they suddenly were running correct again.
mmf on Jul 1 2009, 04:31 PM said:
I do get a band when using primers for a different gene amplicon that we use in another project and I was using here as positive control.
If you have a working primer pair....include it as positive control in every PCR experiment (so you will know nothing is wrong with your DNA, PCR chemicals and gel). When testing primers or protocols it may save a lot of work to include positive and negative controls everytime. So if your positive control was treated like your sample.....maybe you should re-desogm your primers
mmf on Jul 1 2009, 04:31 PM said:
I would give it one try...if you can exclude the not working primers.
mmf on Jul 1 2009, 04:31 PM said:
R U sure you're not the student?
It's not the tubes..so it must be you.
So that's a 100bp ladder? How big is the largest band 3000 or 5000bp?
gDNA generally runs higher than 25 kbp...and so compared to your marker...your gDNA looks like something is wrong with it...and your gel looks like it has only been run for about 20 min....so the crud in the well might be an amplicon...do you load the entire reaction? if yes...why? successful PCR need only analyze 5ul from 50ul reaction..won't see your gDNA template this way unless you are using way too much template...200ng or less I hope.
Well, I am a postdoc, technically still a trainee. I just started to do PCR a month ago. ![]()
Actually, the student's gel suggests it may be the tubes, but it had other problems so I am running a new PCR comparing tubes vs. plate.
The largest band in my 100 bp ladder is 2072 bp. So maybe there is something wrong with my gDNA, that's a good suggestion.
Run time is 40 min @ 100 V. But my understanding is that it is almost impossible to make such big amplicons by PCR (polymerase wont stay on template long enough).
I load 13 uL, ~ half my reaction.
And we were using 250 ng template BECAUSE we were testing primers and having bad luck with them, trying to maximize everything to get anything first, then optimize for the best primer pair.
eldon on Jul 1 2009, 02:26 PM said:
R U sure you're not the student?
It's not the tubes..so it must be you.
So that's a 100bp ladder? How big is the largest band 3000 or 5000bp?
gDNA generally runs higher than 25 kbp...and so compared to your marker...your gDNA looks like something is wrong with it...and your gel looks like it has only been run for about 20 min....so the crud in the well might be an amplicon...do you load the entire reaction? if yes...why? successful PCR need only analyze 5ul from 50ul reaction..won't see your gDNA template this way unless you are using way too much template...200ng or less I hope.
Where is your gDNA from (mean did you extract it, how or who extracted it how)? If you are new to DNA extraction / PCR there are an awful lot of things which can go wrong! So maybe all your efforts in PCR are useless because your DNA is crap.
Eldon is right when pointing out that your gDNA is way to short compared to your marker. You should try to re-do your DNA extraction and look at the crude extract on a gel. This should look like your PCR product.
And ususally too much DNA is the mistake not vice versa! PCR can be as sensitive as a single copy of your gene (which is less than a pg....). If you can exclude crap gDNA, then try serial dilutions of your DNA for PCR.
Thanks.
For what is worth, I have tested gDNA preparations from others in the lab, that they have used fine in their projects and stored, and i still get the same thick blob.
And by the way, I started seeing the blob when I increased MgCl2 concentration while optimizing conditions for my primers. When my boss saw the blob, she panic and since have had me try different things to get rid of it: temperature gradients, cycle number, tubes vs. plate. But so far she has ignored my initial suggestion about again decreasing MgCl.
Late yesterday I tested the tubes theory and that was not it. Although someone else got a much lesse blob than me in an earlier PCR. Maybe it is just me ![]()
At this point, since I do see my desired amplicon with one of the primer sets, I rather ignore the blob and continue optimizing PCR conditions for that primer set. Maybe the blob will go away. Someone told me to try DMSO, that it may "open up" the DNA and then it will no longer get stuck in the well, maybe I'll try that too.
Thanks all for being a sounding board.
gebirgsziege on Jul 2 2009, 03:09 AM said:
Eldon is right when pointing out that your gDNA is way to short compared to your marker. You should try to re-do your DNA extraction and look at the crude extract on a gel. This should look like your PCR product.
And ususally too much DNA is the mistake not vice versa! PCR can be as sensitive as a single copy of your gene (which is less than a pg....). If you can exclude crap gDNA, then try serial dilutions of your DNA for PCR.
