Contamination in negative control of AFLPs (PCRs) - (May/17/2009 )
Hi all,
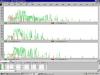
I have begun to see contamination in my negative controls of my PCR reactions of AFLP analysis. I have a negative control for my Digestion and Ligation (contains only the reagents from this reaction), and I create a new negative control for my (first) pre-selective PCR (which contains only the PCR reagents). I run my reactions on a gel, all reactions work well (including the positive control), and there is no smear/bands in the negative controls at this time. I prepare my selective PCRs (a subsequent PCR using products from the first reaction), and also prepare a new negative control with simply the reagents used in this reaction, but also carry through the other 2 negative controls. I analyze the reactions using capillary electrophoresis (labelled primers), and it is here that I see contamination in my negative controls from the digestion/ligation and the pre-selective PCR (up to 250bp long). There are no bands in my selective PCR negative control. I have done all of the instinctive steps to solve this problem, like changing buffers, dNTPs etc. I prepared new dilutions of my primers, and was able to replace some of the stock primers, however, there are still a few primers that I have not reordered yet. I have been extremely careful with pipetting, changing tips, changing gloves etc. Unfortunately I am restricted to working in this one room, and cannot get away from being exposed to Post-PCR samples when preparing my reactions. This issue was not always occuring when starting my AFLPs, and has just crept up on me and I would consider myself a very cautious and attentive worker. I have posted an image of my AFLP profiles. The top panel is the positive control, the middle panel is the negative control initiated from the dig/lig reaction, and the lower panel is the negative control initiated from the pre-selective reaction (the green peaks are sample peaks, they are very strong, I did not dilute my reactions. The red peaks are the size standards, 25bp apart). I plan to reorder the primer stocks that have not yet been replaced. Please help, any suggestions welcome.
Hi again, I wanted to re-upload the file, so that it was smaller. Please see attached.
Thank you ![]()