SDS PAGE of Bovine Serum albumin - Why more than one band? (May/05/2009 )
Hi there.
I have been involved with SDS PAGE for over a decade, and in that time, no one has answered this question to my satisfaction. Why, when running bog-standard BSA on a denaturing SDS PAGE gel, with reducing agents and antioxidants included in the buffers, do you always see more than one band for the protein. The literature molecular weight is 66kDa, for which there is a nice clear band in the gel, but overload the gel with 5ug protein per well, and then you begin to pick up further bands at approx. 180, 210, 300 and 350kDa. Are these extra bands other serum constituents that are co-purified with the BSA in commercial preparations? Or could they be multiplets of BSA? If they are multiplets of BSA then how do they stay stuck together in the denaturing conditions of the gel.
I can find no information directly relating to this on the internet. Anyone who has any ideas or educated guesses regarding this, please put me out of my misery and help shed some light on this mystery. I would be extremely grateful to anyone with anything to say.
Please see attached scans of a couple of gels I ran of several commercial preparations of BSA, one at normal 0.5ug loading, and one overloaded with 5ug per well. These were run on NuPAGE 4-12% BisTris gels with MOPS buffer. It is interesting to note the variation in intensity of the higher molecular weight bands between one source of BSA and another...............................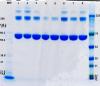

I have noticed similar things and just assumed that the other bands were impurities in the BSA.
Same observation as Bob1...impurities.
bob1 on May 6 2009, 07:30 AM said:
I think the best way is dong WB to see are they BSA dimers or threemers or some impurity! We dont have antibody for BSA to check it for you, unfortunately!
Thanks for all your input guys. I will try and get hold of a monoclonal antibody for a WB. A polyclonal would probably have some specificity for the 'impurities' if it was raised in an animal sensitized with a standard commercial preparation of BSA. I will post the results if my manager will sign my P.O. ![]()
Thanks again.....
paul t on May 5 2009, 01:31 PM said:
I have been involved with SDS PAGE for over a decade, and in that time, no one has answered this question to my satisfaction. Why, when running bog-standard BSA on a denaturing SDS PAGE gel, with reducing agents and antioxidants included in the buffers, do you always see more than one band for the protein. The literature molecular weight is 66kDa, for which there is a nice clear band in the gel, but overload the gel with 5ug protein per well, and then you begin to pick up further bands at approx. 180, 210, 300 and 350kDa. Are these extra bands other serum constituents that are co-purified with the BSA in commercial preparations? Or could they be multiplets of BSA? If they are multiplets of BSA then how do they stay stuck together in the denaturing conditions of the gel.
I can find no information directly relating to this on the internet. Anyone who has any ideas or educated guesses regarding this, please put me out of my misery and help shed some light on this mystery. I would be extremely grateful to anyone with anything to say.
Please see attached scans of a couple of gels I ran of several commercial preparations of BSA, one at normal 0.5ug loading, and one overloaded with 5ug per well. These were run on NuPAGE 4-12% BisTris gels with MOPS buffer. It is interesting to note the variation in intensity of the higher molecular weight bands between one source of BSA and another...............................
A variety of techniques are used to fractionate serum during the preparation of albumins. Standard practices include heat-shock and/or cold ethanol precipitation. These methods always result in some denaturation and aggregate formation. Even chromatographically purified commercial BSA usually uses pasteurized serum as starting material.
The aggregation of albumin (both reversible and irreversible) is of great interest to the producers of clinical blood products.
Since the mid 1940´s a large number of papers have been published on albumin polymerization principally on HSA but also using BSA as a "stand-in". These have utilized a wide variety of techniques including gel electrophoresis, differential scanning calorimetry, light-scattering etc.
I certainly agree that the minor bands on you gels are most probably contaminants. Some of these may be present in the BSA preparations covalently bound to the BSA via S-S bonds. Most BSA preps contain 1 - 5 % gamma globulins . But on a reduced gel these would be seen as heavy and light chains >50 kD.
However, in the majority of your BSA preparations the additional bands represent probably >10 % of the total protein. These are certainly BSA or it`s polymers. Otherwise the BSA you are buying would be very impure!!
Multimerization of BSA has been reported that is irreversible even after DTT treatment. This appears to be involve the interaction of a 6Å cleft around cys-34.
The apparent differences between the various BSA preparations could reflect different levels of production-related denaturation.
BSA that is used in gel calibrators has been purified by gel-permeation in order to remove aggregates. Try running a BSA prep on a calibrated Superdex-200 FPLC column. Pool the "monomer fractions", and run this on a gel. One major peak!
See for example:
BRAHMA A et al. Characterization of a dimeric unfolding intermediate of bovine serum albumin under mildly acidic condition. Biochimica biophysica acta. 2005, 1751: pp. 159-169
An interesting question.
Hope this is useful.
klinmed on May 6 2009, 01:25 PM said:
paul t on May 5 2009, 01:31 PM said:
I have been involved with SDS PAGE for over a decade, and in that time, no one has answered this question to my satisfaction. Why, when running bog-standard BSA on a denaturing SDS PAGE gel, with reducing agents and antioxidants included in the buffers, do you always see more than one band for the protein. The literature molecular weight is 66kDa, for which there is a nice clear band in the gel, but overload the gel with 5ug protein per well, and then you begin to pick up further bands at approx. 180, 210, 300 and 350kDa. Are these extra bands other serum constituents that are co-purified with the BSA in commercial preparations? Or could they be multiplets of BSA? If they are multiplets of BSA then how do they stay stuck together in the denaturing conditions of the gel.
I can find no information directly relating to this on the internet. Anyone who has any ideas or educated guesses regarding this, please put me out of my misery and help shed some light on this mystery. I would be extremely grateful to anyone with anything to say.
Please see attached scans of a couple of gels I ran of several commercial preparations of BSA, one at normal 0.5ug loading, and one overloaded with 5ug per well. These were run on NuPAGE 4-12% BisTris gels with MOPS buffer. It is interesting to note the variation in intensity of the higher molecular weight bands between one source of BSA and another...............................
A variety of techniques are used to fractionate serum during the preparation of albumins. Standard practices include heat-shock and/or cold ethanol precipitation. These methods always result in some denaturation and aggregate formation. Even chromatographically purified commercial BSA usually uses pasteurized serum as starting material.
The aggregation of albumin (both reversible and irreversible) is of great interest to the producers of clinical blood products.
Since the mid 1940´s a large number of papers have been published on albumin polymerization principally on HSA but also using BSA as a "stand-in". These have utilized a wide variety of techniques including gel electrophoresis, differential scanning calorimetry, light-scattering etc.
I certainly agree that the minor bands on you gels are most probably contaminants. Some of these may be present in the BSA preparations covalently bound to the BSA via S-S bonds. Most BSA preps contain 1 - 5 % gamma globulins . But on a reduced gel these would be seen as heavy and light chains >50 kD.
However, in the majority of your BSA preparations the additional bands represent probably >10 % of the total protein. These are certainly BSA or it`s polymers. Otherwise the BSA you are buying would be very impure!!
Multimerization of BSA has been reported that is irreversible even after DTT treatment. This appears to be involve the interaction of a 6Å cleft around cys-34.
The apparent differences between the various BSA preparations could reflect different levels of production-related denaturation.
BSA that is used in gel calibrators has been purified by gel-permeation in order to remove aggregates. Try running a BSA prep on a calibrated Superdex-200 FPLC column. Pool the "monomer fractions", and run this on a gel. One major peak!
See for example:
BRAHMA A et al. Characterization of a dimeric unfolding intermediate of bovine serum albumin under mildly acidic condition. Biochimica biophysica acta. 2005, 1751: pp. 159-169
An interesting question.
Hope this is useful.
This is really useful. Thankyou Klinmed.
