Creating empty vector by self-ligation - (Apr/06/2009 )
Common....you got to digest it. Homebrew is right.
There is a huge difference, the supercoiled form runs lower.
T C on Apr 16 2009, 12:19 PM said:
In that case, just repeat it once.
Best,
TC
chijing on Apr 15 2009, 11:36 AM said:
Does the amount of DNA i used for transformation affect the result?
Initially i used 6ug of plasmid DNA for double digestion and i assume i lost some during the gel extraction process.
I used only 10ul (one third) of the DNA eluted from gel extraction kit for religation.
then, 5ul ligation mix to be transformed into 100ul DH5alpha competent cells.
Will it be too little amount of DNA?
Hey,
You may have already got the clone, just digest the plasmid as Homebrew suggests and run it on a gel.
I have observed that as I increase the amount of DNA for transformation, the number of colonies obtained decrease...I guess its the salts which interfere.
Even a small amount of DNA is enough for religation.
I setup a 10 ul ligation and use everything for transformation.
Hope it helps.
Best,
TC
chijing on Apr 17 2009, 10:56 AM said:
Initially i used 6ug of plasmid DNA for double digestion and i assume i lost some during the gel extraction process.
I used only 10ul (one third) of the DNA eluted from gel extraction kit for religation.
then, 5ul ligation mix to be transformed into 100ul DH5alpha competent cells.
Will it be too little amount of DNA?
I have already tried using Sal 1 to cut the circular plasmid at one site to linearize it. but it's kinda dissapointing..i saw two bands..one is of size around 3.7kb and one is between 1500-2000bp.
T C on Apr 17 2009, 04:34 PM said:
You may have already got the clone, just digest the plasmid as Homebrew suggests and run it on a gel.
I have observed that as I increase the amount of DNA for transformation, the number of colonies obtained decrease...I guess its the salts which interfere.
Even a small amount of DNA is enough for religation.
I setup a 10 ul ligation and use everything for transformation.
Hope it helps.
Best,
TC
chijing on Apr 17 2009, 10:56 AM said:
Initially i used 6ug of plasmid DNA for double digestion and i assume i lost some during the gel extraction process.
I used only 10ul (one third) of the DNA eluted from gel extraction kit for religation.
then, 5ul ligation mix to be transformed into 100ul DH5alpha competent cells.
Will it be too little amount of DNA?
Is Sal I unique?
This is weird. Paste the gel if you can and check with another enzyme. However, do start the whole process again.
Best,
TC
chijing on Apr 17 2009, 06:07 PM said:
T C on Apr 17 2009, 04:34 PM said:
You may have already got the clone, just digest the plasmid as Homebrew suggests and run it on a gel.
I have observed that as I increase the amount of DNA for transformation, the number of colonies obtained decrease...I guess its the salts which interfere.
Even a small amount of DNA is enough for religation.
I setup a 10 ul ligation and use everything for transformation.
Hope it helps.
Best,
TC
chijing on Apr 17 2009, 10:56 AM said:
Initially i used 6ug of plasmid DNA for double digestion and i assume i lost some during the gel extraction process.
I used only 10ul (one third) of the DNA eluted from gel extraction kit for religation.
then, 5ul ligation mix to be transformed into 100ul DH5alpha competent cells.
Will it be too little amount of DNA?
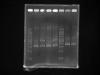
1st lane- 1kb DNA ladder
2nd lane- mini prep extracted plasmid DNA (A)
3rd lane-Sal 1 digested plasmid DNA (A)
4th lane- mini prep extracted plasmid DNA (![]()
5th lane-Sal 1 digested plasmid DNA (![]()
T C on Apr 18 2009, 01:43 AM said:
This is weird. Paste the gel if you can and check with another enzyme. However, do start the whole process again.
Best,
TC
chijing on Apr 17 2009, 06:07 PM said:
T C on Apr 17 2009, 04:34 PM said:
You may have already got the clone, just digest the plasmid as Homebrew suggests and run it on a gel.
I have observed that as I increase the amount of DNA for transformation, the number of colonies obtained decrease...I guess its the salts which interfere.
Even a small amount of DNA is enough for religation.
I setup a 10 ul ligation and use everything for transformation.
Hope it helps.
Best,
TC
chijing on Apr 17 2009, 10:56 AM said:
Initially i used 6ug of plasmid DNA for double digestion and i assume i lost some during the gel extraction process.
I used only 10ul (one third) of the DNA eluted from gel extraction kit for religation.
then, 5ul ligation mix to be transformed into 100ul DH5alpha competent cells.
Will it be too little amount of DNA?
Hey,
There is no difference.....looks like yr enzyme has gone bad or there is no Sal I site and thus its contamination. Did you chk with another enzyme?
Best,
TC
Hmm..do u mean that my competent cells are actually contaminated?
T C on Apr 20 2009, 03:17 PM said:
There is no difference.....looks like yr enzyme has gone bad or there is no Sal I site and thus its contamination. Did you chk with another enzyme?
Best,
TC
Thanks to those who helped me in this post.
I have successfully created my empty vector!!yippie!! ![]()
Hi,
Could you please share with us how you solved your problem?
Regards
GH
