Troubleshooting Digest, ligation, transformation - (Mar/17/2009 )
I've been working on trying to clone this insert for 2 months now with no success! If I don't get this to work, my PI is going to kick me out of the lab!
I'm using a pCR4 topo vector. I have a G418 tag along with my insert. My original plan was to just cut out the insert and flip its orientation (both ends EcoRI). When I couldn't get that to work, we decided to just cut out the insert and religate the vector without it. I can't get that to work either!
At first I thought that my EcoRI wasn't cutting. But it looks like it does on the gel. I gel extracted the vector, added ligase, and transformed it. I am getting the same amount of colonies on my negative control plate (with no ligase) as I am on the ligation plate. Which leaves me to believe enzyme isn't cutting.. although it looks ok on gel. Suggestions anyone?
Help please!!
Thanks
Could you be a little more specific? You are cloning a fragment out of your vector? Using Eco you cut out your fragment and try to ligate the backbone vector?
I have a variety of mutants that I need to insert into my vector. I figured the best way is to have the vector with just the G418 tag. So, I cut out the mutant insert and tried to ligate the vector back together without it.
What other details do you need?
Thanks!
Ok just making sure I understand you... ehm cut your agarose gel isolated frament again with Eco to make sure everything is cut. Try to make the negative control really negative!
Or transform after ligation with trean enhancer to gain more colonies?
Here, I just did another digest and it doesn't look pretty! I don't know how to get a pic on here.. lets see if this works..
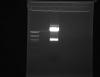
4.0 ul DNA (1 ug)
0.5 ul EcoRI
1 ul 10x buffer that came with enzyme
4.5 ul water
can't see anything but cutting 1 ng DNA with so much enzyme is probably affecting the specificity!
Try cutting 1µg with 0.5µl enzyme in a solution of 20-30µL could be helpful?
sorry, 1 ug cut with 0.5 ul enzyme!
Hi,
how big should your vector and insert be? I can see cut vector otherwise why would you have 2 bands? If i was you, i would try to digest less because the upper band (guess that is your backbone) is quite strong and might contain cut and uncut plasmid and then you would get results like yours...
Stardust
