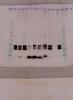
Lower band detected after alanine mutation - (Aug/27/2018 )
Hi all,
after a serial of alanine mutation on some region of interest of a protein, i noticed substitution of alanine of some amino acid will lead to lower band intensity in western blot. But with higher background band at the lower size. It seems for me there's some degradation of these samples. But i supposed the protein was prepared simultaneously and equally. And the experiment repeated twice with same results. Below are the attached pic.
Anyone knows why some sample will have higher intensity of unspecific band than the others?
Possibly the stability of the protein was affected by the mutations? I assume this is a denaturing gel, so it should not be a folding issue affecting the antibody binding. Anybody else got any ideas?
It could be a translational thing, where the translation is being halted at some point as a result of the mutations. How are the proteins being produced? How are the mutations being introduced?
What do all the lanes on the membrane represent?
Yes it's denaturing gel.
The band on the rightest side is the control (wild type protein), the rest of the lanes are mutants, each with a alanine substitution of amino acid at different point of the protein's domain. The mutation is introduced into an expression plasmid via site directed mutagenesis, and transfected into 293T cells. Total protein are extracted from cell lysates.