Viscous plasmids and transfection issues - (Jan/09/2018 )
I have a question, that feel way to basic, but since I changed the workplace, things are done different here and sometimes they encouter some weird problems with plasmids used for transfections. Like they see some "ageing", plasmids that are several months/years old do not work and/or are very viscous, which they fix with boiling.
I do some procedures differently, but still, there are some things that are odd.
Plasmids are around 8 kb, nothing special, and grown in standard E.coli in maxiprep (100 ml) size, yet isolated with a filter-column based midi prep (up to 50 ml of high-copy plasmid) kit from Invitrogen (PureLink), which should be pretty good one. I know that, but there are not any apparent issues with the fact. DNA is high yield (~ 360 ug overal), around 1.9 260/280 ratio, I try not to overdry and dissolve in TE with hour on 37. Mine are all single clones, but others may skip plating and probably vary in their isolation procedures as well (I mention it only since I'm using their batches to make my plasmids from).
Some of the plasmids are really gelly, eventhough they are all usually diluted to around 1 ug/ul. But I have no info about the origins.
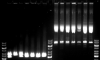
I have only one plasmid, that failed to transfect but I suspected some mutation/recombination event from the mixture or who-knows-what. So I made a digestion of 1ug with two RE in a 50ul volume and pattern seemed different in the first. The other enzyme didn't worked, so I mostly seen undigested plasmid around 8 kb (possible reason is a problem buffer, since the enzyme have been seen to digest in a different one).
But what suprised me was a huge bright smery band with a size much higher. First obvious culprit was bacterial DNA, but on the digested sample set there was nothing like that. These were all my own plasmids, all except the third in each set are around 6 months old (kept frozen), the 3rd is one month old.
I can't rule out some effect of the buffer but it seems like really undissolved DNA. The 8kb bands themselves were having the crystal-like spikes, that are indicative. Still, It surprised me how huge (or retarded) the upper smear was. The DNA should be more or less pure, and I never seen plasmids do things like this when I put them on gel. The concentration (1-2 ug/ul) is also not as huge.
On pipetting, they seem a bit viscous (I need to take care to wait with the tip inside, or the liquid gets "sucked" after I take it out) but not gelly or sticky like gDNA of this concentration is sometimes.
I still wonder, what happends there, if the boiling solves it right (biling would destroy most supercoiled DNA in there, but hard to say if there is any at all) or if there may be some other problem. Also I would appreciate if you could share whether you thing this is a common thing or not, it's not in my experience, but I mostly worked with plasmids for different uses.
I'm also interested due to problems encountered by others, and from a basic curiosity, these same ones work OK, by now.
Pretty weird. Our plasmids were 4 days in a freezer without electricity during hurricane Maria and they are working fine. Do they freeze and thaw the same vial repeatedly instead of prepare a working solution? Its the only thing that I can thought about a DNA that stops working. The viscosity could be too much salts in the buffer or too much DNA? Do your own TE from scratch just in case that someone prepared wrongly the TRIS-HCL and/or the EDTA. Check the DNA concentration in other lab spectrophotometer.
I can see from the gel that you are loading a lot of DNA per well... really, it is often true that "less is more." I usually do 10 ul restriction digests with about 100-200ng per rxn max, unless I want to visualize some tiny fragment, in which case I digest more and accept that the larger bands in the digest will smear. You will get much neater-looking, and easier to interpret gels. I don't think the age of the DNA should be an issue, but if you keep DNA very concentrated (over 500 ng/ul) It often comes out of solution when freeze-thawed, and it is better to dilute it a bit for long term storage. That should prevent the goopiness. Also I would avoid boiling to "fix" the goopiness- too much boiling will eventually result in permanent denaturation of the plasmid DNAs, and this kind of material can be refractory to restriction digest. Gently, gently. Heat to 65 degrees C or so, don't boil!
And by the way, you are doing the correct thing by isolating a single colony to start your preps- what your colleagues are doing by skipping this step is bad science.