Interpreting phospho-Akt Immunoblot in insulin treated mice - (Dec/08/2016 )
Hi,
I'm currently investigating insulin resistance in male and female mice fed a high fat diet.
I am using blunted activation Akt-Ser473 as a marker of insulin resistance.
In this experiment mice are treated with (or without) insulin before being sacrificed, lysates are generated and blotting for total Akt or pAkt.
Using the anti-Akt antibody I see a series of bands at the predicted molecular weight (60kDa). Blotting for pAktSer473 I see two series of bands, one at 60kDa, the other around 75kDa (see image). pAkt is predicted to be 60kDa however the pattern of what I expect to see in insulin treated mice appears at 75kDa.
Once of my colleagues thinks that the bands at 75kDa are non-specific and that pAkt is at 60kDa, while another one of my colleagues thinks that the bands at 60kDa are Akt while the pAkt bands are at 75kDa.
Can someone who has worked with pAkt westerns blotting provide some insight?
Thanks!
Phil
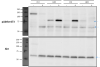
I haven't worked with pAKT, but simple science will tell you what you need to know... phosphate groups are about 30 Da (not kDa) so you would need to add 30 or so to get a 1 kDa difference in mass. As you already know that your protein is running at 60 kDa, it is very unlikely that adding 10ish (I think this is correct) phosphates would suddenly cause it to run at a much higher mass.
Thank you for your explanation. As the pattern of the non-specific bands running at 75kDa is the pattern I was expecting to see at 60kDa, I was note sure would the phosphorylation of the protein affect the migration of the proteins in the gel.
Thanks again!
Phil
