His-tag protein not binding to resin - (Jul/31/2015 )
Background Information:
My goal is to purify a 37kDa His-tagged protein that is expressed by BL21 E. coli cells. Initially I had followed the directions included in the HisLink Spin Protein Purification kit by Promega. The protein is being expressed (confirmed by SDS-PAGE) but is refusing to bind to the Nickel resin. In every of my past attempts, SDS-PAGE shows that a small amount of the His-tagged protein binds to the resin, but the majority of protein that does bind ends up coming off during the 1st washing. A very small amount is recovered after eluting with imidazole.
This led me to suspect that maybe the protein is bind too STRONGLY to the resin, so that it appears faintly in the 1st wash and the elution, however it is mostly still on the resin. I tried imidizole concentrations up to 600mM (elution buffer) and still no difference.
Past attempts for purification:
*All attempts included binding/washing buffers with NO imidizole at pH 8.0
*The kit is brand new (new resin, buffers, etc.)
1) Varying NaCl concentration in binding/wash buffers (0mM, 200mM, 600mM)
2) Denaturing conditions (8M Urea)
3) 100mM Tris binding/washing buffer (usually I use 100mM HEPES)
I don't even use the filter column included in the kits to do the washing/elution anymore. I have found protocols where pelleting the resin and transferring the supernatant is mentioned, and I'd rather save the filter columns for when I actually have a sample worth purifying through filter.
Possible actions:
I am experimenting with using a high concentration of culture per purification. I have been using about 15mL of liquid culture per run, thinking about bumping it up to 100mL (suggested for low level expression proteins). Maybe an even higher imidizole concentration in the elution buffer, 1M?
I tried to be as detailed and concise in sharing my thoughts but probably missed most of the important bits. This has been a very frustrating journey...I will upload SDS-PAGE gel pics soon my SD card port is busted.. Any suggestions are greatly appreciated. Thank you.
Make sure there is no EDTA in your samples and buffers applied to the column. It will strip the nickel (or cobalt) from the column.
how many his residues in the tag? minimum recommended is 6 otherwise it may not bind well.
To confirm expression are you blotting with a His tag antibody? Or did you stain with something non-specific (coomassie, silver stain, etc) and see a band at the right size upon induction?
Why are you using denaturing conditions? Are you trying to isolate and refold from inclusion bodies?
I'm 100% sure that there is no EDTA in any solution used. There are six His residues on the tag, the plasmid is pET-14b and carries a N-terminal His-tag. Recently I have extracted the plasmid and will sequence it to double check.
I have only confirmed expression by SDS-PAGE and staining with coomassie. One can see that the protein is expressed upon addition of IPTG, there is a distinct band. I had assumed that the protein may be forming inclusion bodies or that the His-tag is not exposed to allow for purification. Denaturing seemed the way to go...but it wasn't.
Today I'm trying 1M imidizole. Maybe the protein is binding and the problem is that it won't let go of the resin..
Perhaps your protein has the 6His region out of frame, or it is missing in your plasmid. Have you sequenced the plasmid? Do you know that the 5His region is in-frame and being translated properly?
I plan to send off the plasmid for sequencing tomorrow. It's strange that I am actually hoping that the sequence is messed up...at least then I would have my answer. Thanks so much for the replies, much appreciated. I'll let you know how sequencing goes..
It seems like I have succeeded in purifying this stubborn protein, somewhat by accident. My most recent purification involved using a large amount of culture, although I can't say exactly how much because the centrifuge in lab is....well, not reliable at pelleting cells. Approximately, 100mL I would say of liquid culture that was subjected to...
1) Overnight culture introduced to fresh LB broth (1mL of overnight culture spawned to 99mL of fresh media w/ no antibiotics)
2) Two hours of grow time at 37C.
3) Three hours of induction (IPTG) grow time at 30C.
4) Pelleting followed my protein purification.
...So that was the induction methods.
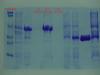
It seems that the protein was binding to the nickel resin, the issue was that it was binding to strongly. I added imidazole to my "purified" protein solution that still contained the resin and the desired protein became apparent by SDS-PAGE. The imidazole concentration was ~2.5M.....which is pretty high. When imidazole concentration was 1M, no protein was apparent by SDS-PAGE.
I will take a picture to share, once I make a gel that is very pretty and good to share (gotta find the ladder first...). I hope that I actually have this protein and I am not mistaken...
Hello, I just wanted to follow through with some pictures and some closing statements. It turns out that 1M imidazole was sufficient to elute the His-tagged protein, however some protein still remained bound and was eluted with a 2M imidazole elution buffer (see second pic). The determining factor though was that the protein had to be denatured to be purified (Pic#1 native and pic#2 denatured). Now I just have to renature it...
If my pictures are not clear and you would like for me to explain further, please post.
Thanks y'all for the help!!!!

You could also look at moving the his tag to the C-terminus and see if you are able to purify it without having to denature it.
