Can I snap freeze my tissue (liquid nitrogen) in Lysis buffer for later RNA extr - (Jan/13/2015 )
Due to having to quickly collect many tissue samples from hyphal mats for RNA extraction during a time-course experiment, I had to freeze my tissue samples quickly so that I could collect RNA at a later date. I quickly collected the hyphal tissues, added them to lysis buffer with 2-mercaptoethanol, and froze them in liquid nitrogen then stored them at -80C.
I am worried that because I did not homogenize the samples in the lysis buffer before freezing and storing them, that I will have ruined my samples for RNA extraction. Would it be sufficient to thaw them in additional freshly prepared lysis buffer when proceeding to do the RNA extraction using the TRIzol PureLink kit? The tissue samples have only been stored at -80C so far for less than a week. I know a 1:10 lysis:TRIzol wil be needed to remove the guanidine isothiocyanate from the lysis buffer.
I am sure you will be okay, because I have done this several times with tissue pieces and stored them for up to a few months with successful qRT-PCR and microarrays later. I remember I just added Trizol to the tube and let the whole thing thaw with occasional vortex zaps.
Time-course experiments. Now I remember where I got that grey hair.
Unfortunately I can't add the TRIzol immediately after it thaws - due to the difficult nature of my samples (like dealing with plant material), I have to homogenize with silica spheres (I use the Precellus homogenization instrument setting 6.5, 45 s shake, 2 times, with 15 s pause and keep everything as cold as possible) before proceeding to the TRIzol extraction with chloroform after. That's where the 1:10 lysis buffer:TRIzol ratio comes in. I'm worried that I have too much tissue for the amount of lysis buffer I froze my samples in. I'll try my best to thaw in additional buffer and then work quickly to only take a smaller amount of frozen tissue before proceeding. I had previously cut smaller tissue samples before freezing, but I didn't have time here, and I thought that it would disrupt the tissue less while still protect the tissue from RNAses.
EXCELLENT RNA quality and yield!!!
The amount of hyphal mat tissue I added to 600 ul of lysis (Invitrogen, PureLink RNA MIni kit) buffer with 2-mercaptoethanol was about the size of a chewed piece of Excel gum. When I went to thaw it today I made to add extra freshly prepared lysis buffer to the top of the tube as it thawed and vortexed it a couple of times during the process. I did flash freeze once more before proceeding with my protocol. Everything was great! So much stress....
Thank-you CPRES!
You are the best, soldier!
You know your one little update will help legions of budding scientists for ages to come, Emily.
From what I saw today with the nanodrop, I think my RNA is of good quality, but perhaps there is something wrong with it... I believe my ratios may be too high. This was diluted in water, using the RNA extraction method I've described throughout this post. I get the following ratios:
260/280 = 2.27
260/230 = 2.7
yield = 1255.35 ng/ul
I'm attaching the plot and report from a screen shot.
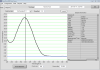
Everything is Ok! I ran this sample on the Agilent Bioanalyzer this afternoon, and it came back with a RIN of 9.3 - Best one I've ever had! One timecourse sample down, 38 more to go....
