problems with 1D SDS PAGE - (Jul/21/2014 )
Hi
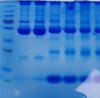
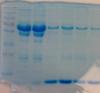
this is a standard 12% SDS PAGE (4% stack) gel run under standard reducing conditions (boiled 95C for 10 minutes in sample reducing buffer)
The samples are blood and plasma.
As you can see the separation is fine until about 80kDa and there are nice defined bands.
After this molecular weight however there are no nice defined bands, just smearing and then those big bands at the bottom.
The molecular weight markers are running fine and I don't think it is a gel issue as I have done this we a pre-made bought gel and with those I have made myself and I am getting the same results.
I'm not really sure what could be happening here
Any suggestions would be great!
Could you run other sample preparations (cell lysates or otherwise) alongside, to see if the gels separate out proteins successfully from other sources?
To be honest, there doesn't appear to be a problem with the gel at first glance, but I rarely run these types of samples. It is somewhat odd that nothing fills in those gaps, but it may be completely consistent with your samples and their preparation. For example, I would expect plasma to have distinct banding pattern as compared to total cell lysates.
Do you have an example of what your gels are predicted to look like? Perhaps this is the normal result for blood and plasma - Like I said, I'm not experienced with these samples, but perhaps others here are.
your "problem" may be due to the acrylamide percentage (12%). the higher molecular weight proteins are bunched up while the lower molecular weight proteins are more widely separated (look at your standards).
you can try a lower percentage gel (eg 10%, 7.5%) or a gradient to obtain more regular banding patterns.
