smear in SDS PAGE - (Jul/19/2013 )
My
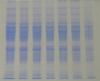
In my view, what you are thinking is correct, it is too much of protein. Is it the lysate from liver? Since lysate will have lots of protein, you can't resolve them on 1D PAGE, that is why it will look like this. And in comparison to muscles , liver will be having much more protein. For confirmation, you can try resolving this in bigger dimension of 1D gel.
first thought:
smearing is often caused by aggregation and/or degradation of the proteins in the sample. you have to ensure complete denaturing by sds/bme prior to loading. extended boiling time may make matters worse. you can try incubating at 60-70C for 10-20 minutes.
you should also clarify the sample, after incubation, by filtration or centrifugation, to ensure that there is nothing insoluble applied to the gel.
if the problem is not the condition of the protein then:
liver tissue contains more lipids and carbohydrates (glycogen) than muscle tissue. one or both of these may be the cause of your problem.
Thank you all for advice.
My loading sample surely gad sds/bme. However, I alway boiled loading sample at 100C in 10 mins, and got good result in the past. However, I will try to incubate at 60-70C and centrifugation before use.
My sample is liver of castrated cattle and have more lipid than normal, but I also get the same problem with the control (normal cattle). When I extraction I tried to avoid lipid layer, and I think I did my best ( I also have the same experiment with muscle contain a lot of intramuscular fat, and I got good result).
In addition, the most smear region is have b-actin and b-tubulin, which I use as control, I really hard to get these band while I can get almost my target. That why this smear is a big problem.
Can you suggest me some ways to decrease lipid/ carbohydrate in my sample? Should I change the lysis buffer?
you may be able to eliminate free lipid and carbohydrate by precipitating the protein with tca.
10 minutes at 100C may lead to aggregation of proteins, even in the presence of sds/bme. if you need to incubate more than 5 minutes then you should do it at lower temperature.
