Wierd Bands after PCR....Confused - (Mar/27/2013 )
Hiii..... As soon as i received my primers i standardized my primers and it was ok at tm-53. But when i tried again after few days there are no bands i tried increasing the Tm to atleast 58 and decreased the Tm till 52 still no bands or just two strange bands lesser than 100kb- possibly primer dimers. So to cross check i ran my already standardized B-actin too but that seems to producing normal expected bands. What could possibly be going wrong. Please help. In the picture attached below the first being the ladder, 2-4 lanes represent B-Actin. The other lanes represent my various other genes.
Thank you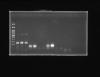
Did lot mastermix or any other component change?
I use a ready PCR mix .. One PCR from Gene Direx.. but as my B- Actin was working i thought not much to do with the components.
I have a few questions and comments to try understand what's going on, and try to help you.
Did your b-actin looked the same as in this picture the time that "worked"? I mean, you seem to have a lot of primer dimer (bright bands at the bottom of gel) compared to what I assume is your specific product (a lot lot fainter). I would not call that a "working" PCR much less would I use it as standarised.
Also, did your other genes show the bands at the bottom in the PCRs that worked? Again, if they did I would not consider that a working reaction.
If your reactions looked like this before I think they need more optimisation to call them "standarised". If, on the other hand, they did work fine (no primer dimer), I'll say you either have a problem with your template or some of your reagents as bigudukaz suggested.
A few things you can play with aside from annealing temp to optimise your reaction are:
<*>Primer concentration: I think it needs to be lower, in view of how much primer dimer you have. If your previous "working PCR" did not show this primer dimer, this might not be the issue.
<*>Template concentration (what is your template? is it stable?). You are either using too little template, and if it is not very stable this time the primer dimers have "win", or you are using too much template and it has some inhibitors that are messing with your reaction. Should be easy enough to try a reaction with more template, and another one with less (dilute your template so the possible inhibitors are diluted too). For PCR usually less is more, if you need tone of template for it to work, is not really working.
<*>MgCl2, DMSO.... varying concentrations of this can help get rid of primer dimer an favour your specific product. I don't know how easy this will be if using a ready PCR mix, but should be doable.
Hope this helps.
Dear almost a doctor and bigudukaz.. Thanks a lot for ur suggestions.. The B-Actin when originally it worked.. there were no primer dimers in it.Even theother primers did not have primer dimers. It looked normal. My confusion is can the Tm of a primer change from time to time and should i try different tm.. or is this something else.. I use a 10 uM concentration of primer and the template is an RT-PCR product 10 ng/ul final concentration. And as my B-actin is working perfectly at that concentration seems to be sufficient for it, i m very confused. I think but as u suggested i have to reduce the template concentration.
Thanks again..
Tm doesn't change.. more likely the PCR equipment itself is not exact with the temperatures.
The very worst thing that could happen - degradation of the primers. loss of couple bases of the primer will lower the Tm a lot..
As you use RT-PCR product then experiment cant produce unspecific products - you add the very same template as the product will be. You should try running PCR with genomic DNA or cDNA.
Are the primers newly designed? As sometimes theory doesn't work hand in hand with practice (not the Tm wise).
Yes the primers are newly designed. Actually i confirmed their specificty with Vector NTI and everything seemed OK.. The machine also maybe fluctuating but not more than one degree + or - i think. But relatively worked ok for my previous experiments.
![]() ...Just hit the new low tried gradient PCR, Tried decreasing the primer concentration, increased Template, added DMSO.. still no hope..Any other options u guys coz im desperate..
...Just hit the new low tried gradient PCR, Tried decreasing the primer concentration, increased Template, added DMSO.. still no hope..Any other options u guys coz im desperate.. ![]() Should i give up and buy new primers???...
Should i give up and buy new primers???...
well seems you have tried everything... the only primers that work are B-actine. it suggests that the problem is in the primers themselves or the template. When you got the primers - were they liothilized or you got them diluted already? cos if they were diluted - there might be no temperature control during delivery, if you have diluted them yourselve out of liothilized stock - there might be a contamination... i always use small purchased stocks of water for this purpose and diluting in laminar box to minimise the chance of azes or something else getting into it.
Yeah i got it as lyophilized powder.. I diluted myself but did not bother with the laminar maybe will try the whole setup again buy new primers.. Thanks a lot for your help and time.. Very useful..
