SDS PAGE - Band artifacts when protein buffer is pH 5.0 and heated - (Dec/15/2012 )
Hi,
Initially I used to purify my protein in Hepes pH 8.0. For certain reasons, now I am purifying my protein with buffer sodium acetate pH 5.0 as final buffer. I observed several bands in SDS PAGE analysis in the end of gel filtration. I figured they could be artifacts of overheating and ran same samples with time of heating as a variable. Interestingly, as I believed, I did not have any unintended bands when sample was not heated but they started appearing as a function of heating time. Another interesting factor is that same protein with buffer as Hepes 8.0, which was heated for 5 mins, did not have any such artifact bands. Please see the attached picture.
I wonder if protein in sodium acetate pH 5.0 can have such detrimental effects when used with sample loading buffer (which is made with b-mercapto ethonol along with usual components). I welcome any inputs explaining/suggesting solution to my problem. Thanks in advance. (I Hope asking questions during weekend is not a bad idea.)
Note: This is a his-tagged protein and Water bath temp was 99 C, if it helps. Those bands cannot be complexing with my protein as band for non-heated sample looks same as heated sample. Sorry for poor quality picture. My lab camera is broke!
Thanks,
Anaivan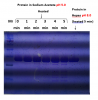
you may have some contamination with an acid protease (eg cathepsins) with your purified protein (may be from some organism living in the protein solution).
maybe acid hydrolysis.
Thanks for your input, mdfenko. This may not be acid hydrolysis as the unintended bands appear only when they are boiled. Otherwise it remains intact.
