Does any one have experience with Zeiss LSM 510 laser scanning confocal microsco - (Oct/11/2012 )
Dear Guys,
I need to use this microscope " Zeiss LSM 510 laser scanning confocal microscope" to get picture of Actin filaments,
However, Actually I couldn't have good pictures, software is so complicated.
Please if any one used this machine before and has good experience in it, share it with me
as I cant do a good Z-series, and I cant have good pictures
Thanks guys in advance
I've used it a fair bit - what's the problem exactly?
I want to visualize actin filament, I got very bad pictures, and I did not get a good stacks.
I am new in using this machine and there is no skillful technician there to help me
I need some advice, as in my lab, no one is helping at all.
I have upload the pictures, could any one suggest some ideas.
https://www.mediafire.com/?wf3ccarxjnnm11a
Perhaps the picture is not good in the beginning,
Please could u tell me ur advic
Thanks in advance
You can attach the pictures directly to your post at BioForum. Mediafire is blocked in many universities and governmental offices. I can't open it.
C:\AIM\Picture%20Naive,%20Control Curtis
Sorry, but I attached it as slm files not picture, to see the stacks as it is.
I have added some photos here too
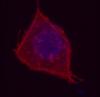
The first one is a wonderful image. What is the problem? I just think your DAPI/Hoechst staining could have been better. How do you permeabilize?
They don't look particularly bad to me. What settings (pinhole, slice thickness, etc) are you using ?
The pinhole is optimal at 1.0 Airy units. Using the palette for this won't work well, unless you set your image up on the red channel. You can easily manually adjust the pinhole using (unsurprisingly) the pinhole control. Anything below 1 will lose signal strength with no gain of resolution, higher will lose resolution but give you stronger signal.
As you are after red channel optimally, you should use the red channel to set the pinhole and thereby determine the slice thickness, then adjust the blue channel accordingly to give you the same thickness slice.
madelingirly on Tue Oct 16 08:30:30 2012 said:
next time incubate at least for 5 min
