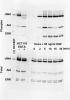
Question with lysis buffer to probe for phospho-cMet receptor - (Sep/21/2012 )
Hi,
I am trying to assay cMet-phosphorylation during HGF treatment in HeLa cells.

I have attached a figure of my attempted assay. The positive control is an HCC827 cell extract (ready to be loaded) graciously sent from a company as I was troubleshooting (hence, I did not lyses and prepare sample for western). As seen in figure 10-30 minutes after HGF treatment show some increase in phospho-cMet (primary AB: Cell signaling Cat# 3077) but may be due to the slight increase in total cMet (primary AB: Cell signaling Cat# 8198). So I also examined ERK phosphorylation and I see a better readout for HGF signaling suggesting the HGF I am using has not gone bad.
Below is my lysis/sample buffer and protocol.
-50mM Tris HCl pH 7.4
-150mM NaCl
-0.5% sodium deoxycholate
-1% TritonX-100
-0.1% SDS
-1mM EDTA
Below are added prior to lysis
-1:100 of Sigma Protease inhibitor cocktail
-10mM Na3VO4
-40mM beta-glycerolphosphate
-20mM NaF
my 5xsample buffer is:
-0.25M Tris HCl (pH6.8)
-10%SDS
-0.5%Bromophenol Blue
-50% Glycerol
-2ml beta-mercaptoethanol.
- I serum starve ~90% confluent HeLa cells for atleast 8 hours - 12 hours.
- Replace media on cells with serum free media + 50ng/ml HGF.
- After indicated time (2-30 min) I wash once with cold PBS (-Ca/Mg)
- add cold lysis buffer and shake briefly to make sure monolayer is covered and does not dry out as I scrape.
- Scrape and place cells in tube on Ice (I flush in and out with pipette as I place in tube).
- Vortex briefly every 10 minutes and place on Ice for a total 30 minutes.
- Vortex and centrifuge 15k rcf for 15 min at 4C.
- Collect supernatant
- Measure concentration (I get around 2-4 ug/ul of protein from 6-well plates without sonication so not too bad). Also I may save samples at this step at -20C if I plan to use samples within 3 days and at -80C for longer storage.
- Bring concentration of samples to equal amounts with 5X sample buffer and the lysis buffer+inhibitors as dilution.
- boil sample 5 min at 95-100C and place on ice.
- Run on gel and transfer.
The main focus of the protocol is try to perform everything as quickly as possible and keep on ice (no ice bucket in hood though). As seen with ERK phosphorylation it seems my stimulation and collecting methods aren't bad.
I'm wondering if my lysis/sample buffer or method of preparing loading samples are bad for examining this type of phosphorylation (membrane associated receptor with epitope specific phopho primary AB)
Any suggestions are appreciated.
Hela are notorious for not responding to things like serum starvation particularly well - you may need to try a different cell line or try a minimum of 24 hours starvation - I have seen people use up to 3 days.
Can't see anything wrong with the lysis and sample buffers.
bob1 on Sat Sep 22 07:31:04 2012 said:
Hela are notorious for not responding to things like serum starvation particularly well - you may need to try a different cell line or try a minimum of 24 hours starvation - I have seen people use up to 3 days.
Can't see anything wrong with the lysis and sample buffers.
I have tried 24 hours of serum starving as well and see the same results. Also, if the problem is not a long enough serum starving period, wouldn't I see the opposite of too high of phospho signals?
Forgot to add - serum starvation works best on cells that are low confluence - about 30-50% is best.
The phospho status of the protein depends on how that protein is affected by cell cycle - some are phosphorylated at particular phases and some aren't. Have you tried synchronizing at different points in the cell cycle?
bob1 on Mon Sep 24 20:45:42 2012 said:
Forgot to add - serum starvation works best on cells that are low confluence - about 30-50% is best.
The phospho status of the protein depends on how that protein is affected by cell cycle - some are phosphorylated at particular phases and some aren't. Have you tried synchronizing at different points in the cell cycle?
I will try serum starving at lower confluence. I usually serum starve 70-80% so that after the 24 hour starving it will reach >90% confluency.
Since the c-Met receptor is activated by HGF which stimulates proliferation, if residual serum is present I would hypothesize that the phospho level would actually be higher than what I am seeing.
I have not tried different points in the cell cycle. I doubt this ligand dependent phosphorylation of the receptor is that sensitive to the cell cycle. Activation and internalization of the receptor occurs within 30-60 minutes after activation therfore I suspect something other than not stimulating cells at an exact stage of the cell cycle.
problem solved! it was the damn antibodies.
I have sent the same image to the antibody company.
Hopefully they will offer a new vial of antibodies free of charge.
Thank you everybody though for you help.
Below is blot done with new antibodies.