What causes surface fluorescence on agarose gels? - (Aug/13/2012 )
perhaps the EtBr wasn't dispersed evenly in the agarose, i.e. more agitating necessary when the EtBr is added?
First it appeared like a film of very tiny condensed drops to me (just like you have when getting stuff out of the fridge when air humidity condenses on it), but after electrophoresis it's not likely
We are very mindful to vigorously swirl the molten gel for several seconds after we add the EtBr.
We see the EtBr disperse throughout the molten agarose.
BTW, we add 25 uL of 0.2 ug/uL EtBr to 350 mL gels.
We also tried adding 200 uL of 1/8 diluted EtBr. ... stil happens ... apparently randomly.
We store the EtBr in the dark. When not in use we store the gels in the electrophoresis chamber in the dark on a cart in walk-in fridge
(we cover with a heavy green plastic grocery shopping basket). Likewise, the 1x TAE contains EtBr and we store i in walk in fridge in dark.
We even arranged so that only 1 person (me) pours gels for 2 months.
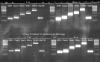
What's frustrating is that the surface fluorescence smudge seems to appear randomly.
Some gels are perfect. Some have surface smudge.
This ought to be a simple thing to figure out ... alas, it's still a mystery.
Do you carefully wipe down the transilluminator between taking photos, making sure to use a wet paper towel or something to wipe away any residue?
I ask because this very same smudging used to happen to a student in our lab, turned out she wasn't wiping the transilluminator after removing her gel so any buffer and moisture from the gel just dried onto the glass. This then showed up on her next photo.
You may not be heating your agarose sufficiently, or for long enough. Agarose takes a while to dissolve, and will clump badly given a chance. Make sure the solution is crystal clear and well mixed.
Thanks to all who have posted replies.
Our gels are thoroughly melted (no "ghosts of undissolved agarose) ... crystal clear.
Just now I posted another thread regarding non-hazardous substitute for ethidium bromide.
