Non-specific bands and fuzzy bands - (Jul/17/2012 )
Hi all,
I'm trying to see whether an antibody targeting a rat protein would cross-react with the zebrafish equivalent and save me a lot of hassle. I've done the Western with different dilutions of the primary antibody (1:100, 1:250, 1:500 -- the recommended concentration is 1:100; used 1:20,000 secondary antibody)) and have run a rat tissue control as well as a zebrafish sample and there's a lot of non-specific bands even for the rat tissue sample (and they are bright!), which doesn't inspire much confidence. Is there any way to decrease the amount of non-specific bands showing up? I blocked with 5% skim-milk powder for 4 hours and probed with primary antibodies overnight (should I cut down on that?). Probed with secondary for 1 hour and developed the blot.
Another thing is that the bands are quite fuzzy. I have no idea why that is. Is there any way to fix that too? I make my own 12% SDS-PAGE gels with 4% stacking, and run them using Tris-glycine buffer at 200V. My transfer buffer contains Tris, glycine, methanol and 0.03% SDS, and I transfer for 1 hour at 100V onto PVDF membrane.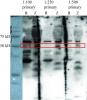
The ways to decrease non-specific binding are - dilute further, run less protein, try different blocking agents, wash more and/or under more stringent conditions (more detergent, lower or higher salt). You can also incubate with the primary for a shorter time.
You bands don't look particularly fuzzy to me, but if you run a bit slower (try 120 V) and perhaps a higher percent gel may help.
