Problems with inhibition? - (Jan/28/2012 )
Hello everyone, I've been having lots of problems lately with my real-time PCRs. I use SYBR green chemistry and I am trying to optimize some primer pairs. I have started with the actin one and after trying several primer concentrations, total reaction volumes, different enzyme mixes, different types of water and even different samples to construct the dilution series, I keep getting efficiencies of 200%. I suspected at first that it might be due to PCR inhibition, but given that after all the different things I`ve changed I get the same results, I'd think is something else. One thing that seems to be constant is that the asymptote of the curve gets higher as the sample gets diluted across the dilution series (that's why I first thought that inhibition might be the problem). What should I do? I've ran out of ideas.
Thanks for the help!
Alex.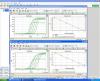
Are you doing RT-qPCR? The RT mix inhibits reaction a bit.
By asymptote you mean the fluorescent level of the plateau? That's normal and doesn't have usually effect on quantification.
What happens to the efficiency if you discard the first dilution (or first two)? The first one have notably different slope of the linear part of the curve, I'd like to see it in logarythmic scale, it sometimes indicates different slope of exponential part, which means decreased efficiency of the reaction.
It normally gets even worse when I discard the first two dilutions. It's RT-qPCR from rat brain tissue. I also tought of the RT mix, is there any purification method to be applied after the RT to prevent this problem?. I'll post the logarithmic plots tomorow, I'm at home and have no access to the right now. Thank you very much.
Alex.
I used to have the same problem. I mistakenly assumed the std curve and the samples being analyzed had to be in the same Ct range. For the std curve to give an acceptable efficiency, you need to use standards that are significantly more concentrated than your samples being analyzed. I generally make my standards by running a qPCR reaction to completion, purifying the product with Qiaquick (about 20ng/ul) and then using this product at 1:100, 1:1000, 1:10,000, and 1:100,000. Often, I have to omit the 1:100,000 sample from the analysis if it is not in line with the other 3.
doxorubicin on Sun Jan 29 17:37:58 2012 said:
I used to have the same problem. I mistakenly assumed the std curve and the samples being analyzed had to be in the same Ct range. For the std curve to give an acceptable efficiency, you need to use standards that are significantly more concentrated than your samples being analyzed. I generally make my standards by running a qPCR reaction to completion, purifying the product with Qiaquick (about 20ng/ul) and then using this product at 1:100, 1:1000, 1:10,000, and 1:100,000. Often, I have to omit the 1:100,000 sample from the analysis if it is not in line with the other 3.
Well, to my knowledge you do need to have standard around the same range (better a bit concentrated) like your samples, I'm curious where did you learn that it should be otherwise?
And what more you need to use the same sample type. Complex cDNA will have different efficiency than very dilluted PCR product.
IMHO it's not about 'getting acceptable efficiency' but measuring the real efficiency of your reaction.
ahmatas:
Other thoughts.. you said SYBR, did you check for primer dimers or nonspecific binding? I suppose you double checked the setting of the standards and the dilution. But what strikes me, is that 223% efficiency is just too much to be caused by inhibition, when there is no visible trend in the curves being closer in the lower dillutions (except maybe for the first one or two).
Thanks for your suggestions, I've lost the count of how many test experiments I've already carried out. I've checked everything you suggest. I have even tried a commercial control assay from Bio-Rad (which amplifies a specific sequence from plasmid DNA), and I get the same results. I attach the previous data in Log scale. I don't know what else to try...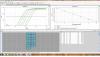
I forgot to say that this only happens with SYBR green, Taqman assays work just fine. I've even tried to use some of the primers that worked with probes and still, an efficiency of 250% is what I get.
What about changing the SYBR mix? If you have the same samples, same machine, same dilution, same calculations and same primers the only thing different is the chemistry.
Yeah I can try that, I have used two different mixes but both of them were from Bio-Rad, I can try another distributor. Thanks again!
hello, i need help , my question is that there r lots of inhibitions in realtime -pcr of hcv rna. most probably in extraction of hcv- rna. i have checked that there is complete removal of ethanol which i m using in extraction and there r no precipitates in lysis buffer so wat shud i do? we r using COBAS AMLICOR MACHINE with roche kits.
