chromatin shearing using Bioruptor sonicator - Water bath sonicator for chromatins shearing (Feb/14/2011 )
Hi everyone,
I'm wondering if there are anyone that has been using bioruptor sonicator to shear their chromatin? Mostly for ChIP experiments. The company said that there has been so many people shearing many different cell lines so it should be easy to find the shearing time for each specific cell line. But I have been googling and searching on pubmed for a while and could never find those infos. I am working with breast cancer cell lines (mostly MDA-MB-468). If there is anyone that has sheared breast cancer cell line before, please reply. Other people are also very welcome to post since any information about this bioruptor sonicator would be useful. We are using bioruptor xl, a water bath based sonicator that can load up to 6 x 1.5 ml microtubes.
Thanks heaps!!!
I made use of the Bioruptor Sonicator for my ChIP experiments. I didn't use a cell line though, it was on the nuclear fraction of neuronal tissue from mice. Nonetheless, my setting were: High, five minutes total, 30s on 30s off; this gave pretty consistent shearing yielding 200-800 bp size fragments. I did crosslinking in 1% formaldehyde for 10 min. I don't know for sure, but my guess is that your crosslinking conditions and sonication buffer will likely be more important than the cell type with respect to determining sonication conditions. Perhaps this will at least give you a place to start. As a general rule I would say you would want to use the least amount of sonication possible to obtain the required fragment size.
Hope that helps.
MM
Mighty Mouse on Mon Feb 14 22:18:37 2011 said:
I made use of the Bioruptor Sonicator for my ChIP experiments. I didn't use a cell line though, it was on the nuclear fraction of neuronal tissue from mice. Nonetheless, my setting were: High, five minutes total, 30s on 30s off; this gave pretty consistent shearing yielding 200-800 bp size fragments. I did crosslinking in 1% formaldehyde for 10 min. I don't know for sure, but my guess is that your crosslinking conditions and sonication buffer will likely be more important than the cell type with respect to determining sonication conditions. Perhaps this will at least give you a place to start. As a general rule I would say you would want to use the least amount of sonication possible to obtain the required fragment size.
Hope that helps.
MM
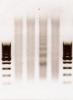
Hmmm, perhaps you will be able to help me out as well. You are the first person I have come across who isn't beginning with cell culture and is rather using tissue extracted from mice. I am attempting to optimize sonication for spinal cord tissue (using brain and liver tissue as controls) and have been having a very difficult time, also using a bioruptor. I have been starting with a 50mg piece of brain, liver, and spinal cord tissue. Homogenizing in either DPBS or a Nonidet p-40 containing cell lysis buffer, Xlinking in 1% formaldehyde, quenching with .125M glycine, then resuspending the cell pellet in 150ul of SDS lysis buffer. I have then been trying sonication periods of 1h (high power, 30s on 30s off, adding ice to the water bath every 10min) and 0.5h. On the attached images, the samples in the first image (3/18) were homogenized in DPBS, and the second image (3/22) we used the detergent containing cell lysis buffer to homogenize the tissues. In each image, the three lanes on the left were subjected to 1h sonication and the three lanes on the right were sonicated for 0.5h. In each set of three lanes, the brain is on the left, liver in the middle, and spinal cord on the right
Well for what it's worth, I cross-link prior to homogenizing. I used the hippocampus from mice, so I would remove the hippocampus, chop it up with a razor blade, then incubate it in formaldehyde for 10 min w/ rocking, then glycine, then I would homogenize etc (I think I've posted the protocol around here somewhere, but I can re-post it if you need). If you are looking at TFs as opposed to histones, I think this could make a considerable difference as you want to lock in that TF on the DNA as quickly as possible before manipulating your sample too much.
I'm not sure what your question is regarding sonication, but 30 min or 1 hr strikes me as a long time, as I did it for 10 min. The bioruptor is pretty powerful, but I'm sure its efficiency depends a lot on tissue type. Although one thing to keep in mind is that adding ice to the water bath is going to decrease your overall sonication efficiency and could very well increase your variability. The ice in the bath will end up absorbing some of the energy of sonication. When I was using the BioRuptor it was stored in the cold room. Between runs I would put ice in the bath to cool the water down for a bit, but I would always remove the ice prior to sonicating so as to keep my conditions as consistent as possible.
MM
Mighty Mouse on Wed Apr 20 23:04:18 2011 said:
Well for what it's worth, I cross-link prior to homogenizing. I used the hippocampus from mice, so I would remove the hippocampus, chop it up with a razor blade, then incubate it in formaldehyde for 10 min w/ rocking, then glycine, then I would homogenize etc (I think I've posted the protocol around here somewhere, but I can re-post it if you need). If you are looking at TFs as opposed to histones, I think this could make a considerable difference as you want to lock in that TF on the DNA as quickly as possible before manipulating your sample too much.
I'm not sure what your question is regarding sonication, but 30 min or 1 hr strikes me as a long time, as I did it for 10 min. The bioruptor is pretty powerful, but I'm sure its efficiency depends a lot on tissue type. Although one thing to keep in mind is that adding ice to the water bath is going to decrease your overall sonication efficiency and could very well increase your variability. The ice in the bath will end up absorbing some of the energy of sonication. When I was using the BioRuptor it was stored in the cold room. Between runs I would put ice in the bath to cool the water down for a bit, but I would always remove the ice prior to sonicating so as to keep my conditions as consistent as possible.
MM
Hey Mighty Mouse,
I am using Bioruptor to sonicate embryo tissues now. I had a very hard time. I used lysis buffer containing 0.1% SDS. I set the Bioruptor as high 30' on and 30' off, total 45 mins. I only got small amount of DNA within 100 bp-500 bp.
And I checked the protocol of Bioruptor. They suggest the optimal concentration of SDS is around 1%. What kind lysis buffer did you use? Could you please give me the recipe?
And if possible, could you please give me your protocol?
Thank you so much!
If you want to enrich for nuclei before sonicating i would recommend doing a cell lysis followed by a nuclear lysis.
YOu can also disrupt tissues first using a dounce homogenizer before lysis.
Cell Lysis Buffer: 5mM PIPES, 85mM KCl, 0.5% NP-40
Nuclear Lysis Buffer: 50mM Tris-HCl, 10mM EDTA, 1%SDS
Mighty Mouse on Wed Apr 20 23:04:18 2011 said:
Well for what it's worth, I cross-link prior to homogenizing. I used the hippocampus from mice, so I would remove the hippocampus, chop it up with a razor blade, then incubate it in formaldehyde for 10 min w/ rocking, then glycine, then I would homogenize etc (I think I've posted the protocol around here somewhere, but I can re-post it if you need). If you are looking at TFs as opposed to histones, I think this could make a considerable difference as you want to lock in that TF on the DNA as quickly as possible before manipulating your sample too much.
I'm not sure what your question is regarding sonication, but 30 min or 1 hr strikes me as a long time, as I did it for 10 min. The bioruptor is pretty powerful, but I'm sure its efficiency depends a lot on tissue type. Although one thing to keep in mind is that adding ice to the water bath is going to decrease your overall sonication efficiency and could very well increase your variability. The ice in the bath will end up absorbing some of the energy of sonication. When I was using the BioRuptor it was stored in the cold room. Between runs I would put ice in the bath to cool the water down for a bit, but I would always remove the ice prior to sonicating so as to keep my conditions as consistent as possible.
MM
This might seems a tad over the top but we designed a water cooler for our Bioruptor with some copper tubing, a paristaltic pump, and a bucket full of ice water. We used the pump to circulate the water in the Bioruptor through the copper tubing in the ice bucket. It worked very well, with the water in the Bioruptor not getting much above 4C after 15min on high.
As for sonication efficiency, one thing we found which helped was to use thin walled tubes like you would use for PCR.
Hi there, I also seek help for my sheering problem from bioruptor.
The problem is that it takes more than 30 min and when it is done, the range of DNA size is too broad I cannot sure it is enough.
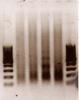
The first image is after 30min shearing. Markers are 100bp interval. 4 lanes in between are 4 different samples.
In this image shearing is long way from the optimal range so I decided to go 15 min more.
And the second image is the result. Most of DNA is in 100~300bp range and I guess it is short. The rest is even longer than 1kb and it is too long.
I followed Magna-ChIP protocol from Millipore. 10^7 mouse primary CD4+ T cells for each shearing sample, 10min x-linking in RT, glycine and cell lysis buffer. The last is custom made, just usual cell swelling buffer. Then in 500ul SDS lysis buffer, 30sec on/off cycle and I refilled ice every 5 minutes. 10^5 equivalent DNA are loaded on gel after reverse X-linking.
Anyone who suffered similar issue I would like to hear anything from you.
Thank you in advance.