additional proteins in eluted protein-protein purification of GST tag fusion pro - (May/07/2010 )
![]()
Hey all
I have been trying to purify my GST tagged protei, that is pGEX 6P-1-rPSPdss-V5 , it has prescission protease site. My problem is that I am getting many additional bands in my eluted protein fraction, have tried many ways, but im not getting my PSP protein in pure form.
my experiment goes like:
Protein expression of GST-rPSP-V5
50 of LB medium is inoculated with BL 21 cells harboring the GST-rPSP-V5 clone. It is incubated at 37 degree Celsius overnight.
2ml of overnight culture is inoculated in fresh 100ml LB medium, and incubated until the absorbance at 600nm reaches 0.8.
Protein expression is induced by addition of 0.1mM IPTG (isopropyl 1-thio-beta-D-galactopyranoside) to final concentration, and incubated for 2 hours.
Cells are harvested by centrifugation at 3750 rpm, for 20mins at 4 degree Celsius. The resulting pellet is washed with 1X ice cold PBS and 1mM phenylmethylsulfonylfluoride (PMSF), centrifuged again and stored at -20 degree Celsius.
The 200ml bacterial culture pellet is resuspended in 5ml of 1X PBS, with 1mM PMSF and Roche protease inhibitor tablets. To the resuspended pellet 5mM dithiotheritol (DTT), 1mM EDTA and 0.25% sarkosyl added.
The solution is sonicated 4 times (10secs of sonication with 10secs of cooling ice), speed 2 with Branson sonifier and centrifuged at 20,000rpm for 20 minutes at 4 degree Celsius. To 1.5ml of sonicated bacterial supernatant, 200ul of glutathione sepharose resin 50% (v/v) added. After incubating it for 60 minutes at 4 degree Celsius with mild agitation, the solution is spinned at 1000g for 1min. The unbound protein fraction is saved for running on the gel. Protein bound to the resin is washed 5 times, with 1.5ml of 1XPBS and 1mM PMSF, spinning it at 1000g for 1 minute. I changed these washing conditions by making the wash volume as 10 times the bed volume of the resin, adding NaCl upto 600mM, and washing the resin with the wash buffer at 4 degree celsius for 10 mins, i.e 10 mins washes, and 5 washes . Even tried adding 5% glycerol to washing buffer, BUT NOTHING AFFECTED THE LEVEL OF PURITY OF THE PROTEIN.After washing, 200ul of 15mM elution buffer containing 50mM Tris, 1mM EDTA, 100mM Nacl, 15mM reduced glutathione, ph 8 is added to the resin. The solution is prepared fresh every time before use. Resin is incubated with the buffer for 60 minutes at room temperature with mild agitation, followed by spinning it at 5000g for 5 minutes. Elution is done once more to strip off most of the protein bound on the resin, incubation the resin second time with the elution buffer for 30 minutes. 10% glycerol is added to the eluted protein, and stored at -80 degree Celsius.
EVEN TRIED ADDING 2mM ATP AND 10mM MgSO4 to the lysate before applying it to the resin, and incubating it at 37 degree celsius, for 10 mins, thinking that one extra band out of many could be DNak protein , which has an E.coli protein, and has high affinity for GST.
PLEASE NYONE LET ME KNOW WHERE CAN I MAKE THE CHANGE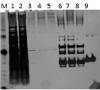
Hello, i am not sure which is the band corresponding your protein in the gel, but i can tell you three things:
-Firstly, i woul make the adsortion of the protein 1 h/RT/shaking or o/n 4ºC/shaking, the binding is less at 4ºC and you should increase the time of contact.
-Secondly. Maybe you can change the ratio seed/resin to avoid contaminants to bind the resin, try 100 or 150 ul of resine or rise the seed to 200 ml or so.
-Finally. I always have found in the recombinant proteins fused to GST degradation products: GST alone mainly. I suspect that there is some sequence target for hydrolisis in the commercial plasmid (my plasmid was pET-42, but some of them share some DNA sequences). Actually, i eliminated a DNA fragment in one construction and the degradation product disappeared completely.
I hope you have good luck!!
dgoyal on May 7 2010, 06:44 PM said:
Hey all
I have been trying to purify my GST tagged protei, that is pGEX 6P-1-rPSPdss-V5 , it has prescission protease site. My problem is that I am getting many additional bands in my eluted protein fraction, have tried many ways, but im not getting my PSP protein in pure form.
my experiment goes like:
Protein expression of GST-rPSP-V5
50 of LB medium is inoculated with BL 21 cells harboring the GST-rPSP-V5 clone. It is incubated at 37 degree Celsius overnight.
2ml of overnight culture is inoculated in fresh 100ml LB medium, and incubated until the absorbance at 600nm reaches 0.8.
Protein expression is induced by addition of 0.1mM IPTG (isopropyl 1-thio-beta-D-galactopyranoside) to final concentration, and incubated for 2 hours.
Cells are harvested by centrifugation at 3750 rpm, for 20mins at 4 degree Celsius. The resulting pellet is washed with 1X ice cold PBS and 1mM phenylmethylsulfonylfluoride (PMSF), centrifuged again and stored at -20 degree Celsius.
The 200ml bacterial culture pellet is resuspended in 5ml of 1X PBS, with 1mM PMSF and Roche protease inhibitor tablets. To the resuspended pellet 5mM dithiotheritol (DTT), 1mM EDTA and 0.25% sarkosyl added.
The solution is sonicated 4 times (10secs of sonication with 10secs of cooling ice), speed 2 with Branson sonifier and centrifuged at 20,000rpm for 20 minutes at 4 degree Celsius. To 1.5ml of sonicated bacterial supernatant, 200ul of glutathione sepharose resin 50% (v/v) added. After incubating it for 60 minutes at 4 degree Celsius with mild agitation, the solution is spinned at 1000g for 1min. The unbound protein fraction is saved for running on the gel. Protein bound to the resin is washed 5 times, with 1.5ml of 1XPBS and 1mM PMSF, spinning it at 1000g for 1 minute. I changed these washing conditions by making the wash volume as 10 times the bed volume of the resin, adding NaCl upto 600mM, and washing the resin with the wash buffer at 4 degree celsius for 10 mins, i.e 10 mins washes, and 5 washes . Even tried adding 5% glycerol to washing buffer, BUT NOTHING AFFECTED THE LEVEL OF PURITY OF THE PROTEIN.After washing, 200ul of 15mM elution buffer containing 50mM Tris, 1mM EDTA, 100mM Nacl, 15mM reduced glutathione, ph 8 is added to the resin. The solution is prepared fresh every time before use. Resin is incubated with the buffer for 60 minutes at room temperature with mild agitation, followed by spinning it at 5000g for 5 minutes. Elution is done once more to strip off most of the protein bound on the resin, incubation the resin second time with the elution buffer for 30 minutes. 10% glycerol is added to the eluted protein, and stored at -80 degree Celsius.
EVEN TRIED ADDING 2mM ATP AND 10mM MgSO4 to the lysate before applying it to the resin, and incubating it at 37 degree celsius, for 10 mins, thinking that one extra band out of many could be DNak protein , which has an E.coli protein, and has high affinity for GST.
PLEASE NYONE LET ME KNOW WHERE CAN I MAKE THE CHANGE
