What could be the cause for this GFP expression pattern...? - (Mar/17/2010 )
When I transfect my cells with empty GFP vector, fluorescence pattern is citoplasmatic and nuclear, as it should be.
But when I transfect cells with my construct (a GFP tagged version at c-ter of my protein of interest, which I suspect to interact with a membrane receptor and is predicted to be citoplasmatic in in silico studies), it seems to have a bright punctate pattern (perhaps compatible with localization at membrane microdomains) along with a quite intense fluorescene in citoplasm and nucleus!!!
Should I suspect an artifactual localization of my fusion protein? Why I found it in the nucleus?
Any suggestion on how I must continue these experiments is welcome.
GFP is a quite large tag and, despite its common use, is often not a good model for this sort of thing - it commonly inhibits normal functions of tagged proteins through steric hindrance. You will probably be better off determining the localisation of your protein of interest with immunofluorescence, using an un-tagged vector for transfection if need be.
It's tricky, you have to try both N-link and C-link to see which one works better.
I add some pictures for clarification. Any idea about what this punctate pattern should mean? It is not present in the mutant construct of the fusion protein...


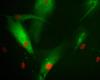
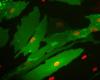
It all depends on the protein features and how you fused them. I have done some fusions and in the pictures 4 and 5 they're just N- and C- fusion and you can see huge difference.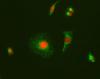
Regarding your punctuate pattern, it may be due to the wildtype has more expression than the mutant
