sample buffer pH - its effect?? (Dec/14/2009 )
Hi
just was curious how does sample buffer pH effect SDS-PAGE profile??
do you all play around with changing its pH ??if so what is the usual range??
the pH of the sample buffer is important to promote stacking of the sample. i wouldn't play with it if it is working (if it ain't broke, don't fix it).

mdfenko on Dec 15 2009, 02:54 PM said:
My team is running antibody on SDS-PAGE (IgG1) and was getting multiple bands under main band… we were thinking of evaluating various parameters in SDS-PAGE and look at its effect. One of the options came to my mind was sample buffer pH (we have never played around with it in our lab, so my curiosity gene got activity to know what its effect will be…trying to “break” it and then “fix” it….
Well so the question still remains open
rick112 on Dec 16 2009, 12:18 AM said:
Well so the question still remains open
raising the pH towards the pH of the electrode buffer will decrease stacking (not entirely because of the stacking gel buffer) and increase band broadening.
lowering the pH may increase stacking up to a point then it may cause the sample to stop (neutralize the negative charge imparted by the sds) until it mixes with the electrode buffer ion front. this may also increase band broadening.
you have to assume that the originators of the various page methods have optimized the buffer conditions (for their samples, at least), but, if you want to determine if they are optimum for your sample then go ahead, knock yourself out.
you may want to try other sds-page formulations (eg weber & osborne, neville,...), laemmli is the standard but not necessarily the best for every purpose (we routinely use neville).
by the way, have you determined if those extra bands were igg or a contaminant? do you always see them? are they new?
for something like an antibody, I would think smaller bands would indicate degradation of your sample...? can you post a pic?
a pic wud surely be nice if u can share.. and the buffer used was reduced or non reducing?? a reducing gel will give u more bands for an antibody anyways as it is all bonded by disulphides!!! Pradeep Iyer on Dec 17 2009, 04:53 AM said: hey rick.. this image might just solve your querry.. as i said tere may be many different combinations possible in an antibody so if you are sure about the purity of your antibody and you have checked with WB tat the lower bands are what u desire, these might be due to your antibody only but still we wud want o see the gel picture. If its too much more then that may not be true!!! hmmm....I'm a bit skeptical here. What could be the reason for color change of Lammli buffer? I was in Blue color whne prepared. Now changed to light red!
I am not sure abt the pH variation ex
I am not sure abt the pH variation ex
hi
i will try to upload a pic...
in one way we tried SDS concentration (we prepared our samples in 1X,2X,4X sample buffer concemtration..not much difference there...although this is not an exact ya to evaluate SDS concentration...)
oh by the way this is NON-reducing gel...
to mdfenko
little curious with Neville and Weber methods...can you if possible pass me protocol or any article about those methods,may give it a try and then see what happens...
thanks every one 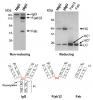
I think that adding varying concentrations of buffer will not solve the problem of the wrong bands. changing the buffering capacity of your system is not a good idea unless you change it everywhere; just adding twice as much buffer won't solve the issue. even if by some miracle you saw what you had hoped to see, I think that your data would not be trustworthy.
have you re-made all buffers and solutions in case there was an error? even if you buy it all pre-made there could have been a dilution error somewhere or something.
