western blot w/ anti-FLAG ab - multiple bands show up (Dec/04/2009 )
Hello everyone,
I performed a western blot with anti-FLAG antibody (Cell Signaling) at a dilution of 1:200 in TBS (0.1%T-20). I expect to find a single band specific to the FLAG epitope tagged to my protein of interest but I ended up getting multiple bands. To make matter worse, I don't see any signal around the expected molecular weight of the my protein (deduced just from amino acid sequence).
So I have a couple of questions:
1. Will using a more diluted anti-FLAG Ab remove those unspecific bands? ( See the picture of the blot)
2. I used a 1:2000 dilution of the secondary antibody? Is that causing unspecific bands?
3. I expect my protein to be running around 75 KDa which is deduced just from amino acid sequence. So is there any way to know if it has a mol wt greater than 75 KDa. Thinking abt PTMs. Btw it is a transcription factor.
4. I used a untagged strain as a negative control (on the far right of the film besides the marker) and it shows faint bands adjacent to those multiple bands. Remember it is way under loaded.
5. Does anyone know of FLAG antibody binding non-specifically? I read somewhere in this forum about that problem.
Thank you. I would be glad to get any kind of suggestions.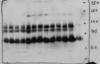
Are you sure you used fairly fresh betamercaptoethanol?
Buddy on Dec 6 2009, 01:13 PM said:
I have been wondering about that. I used a sample buffer containing BME stored at 4 degree for a month. But it had that obnoxious smell of BME so decided to use it. Right now am trying fresh BME to the sample buffer. Thanks for the suggestion.
I would say 1:200 is way to high... ( But I use the anti-flag from Sigma).
I can tell you how I do it. I use it at 1:5000 overnight, and then the secondary 1:10000 1.5-4 hours, and it gives me really nice blots. It is true that if the solution you use is new, sometimes you get background bands, but not big deal.
I saw cross reactivity with gfp and flag, but using high amount of proteins.
laurequillo on Dec 7 2009, 09:48 AM said:
I can tell you how I do it. I use it at 1:5000 overnight, and then the secondary 1:10000 1.5-4 hours, and it gives me really nice blots. It is true that if the solution you use is new, sometimes you get background bands, but not big deal.
I saw cross reactivity with gfp and flag, but using high amount of proteins.
I am having the same problem with nonspecificity of anti-flag however ones from Sigma.Ihave tried monoclonal (get no signal) and polyclonal i see nonspecific band even in cells that don't have the flag vector.I have tried different dilutions of antibody and different dilutions of cell lysates still i get nonspecific signal.which cells are you using to prepare your lysates for western?
I use an anti-FLAG mAb from Sigma at 1:1000 in blocking buffer (either 1 hr at room temp or 4 C overnight). The secondary is goat anti-mouse either HRP-conjugated or biotinylated at 1:15,000, also in blocking buffer (1 hr at room temp). It works great. I do get some nonspecificity using the secondary biotinylated, but that's because the strepavidin-HRP binds a few endogenous biotinylated proteins, not because of the primary or secondary binding nonspecifically. All of my work is done with bacterial proteins.
Based on your image, I'd say that you could dilute the sample more, as well as trying to dilute the antibodies. Do you know for sure that the flag fusion is being expressed?
Is the genome of your organism sequenced? Is there any possibility that the FLAG motif is naturally occurring in some proteins?
rhythmgenes on Dec 5 2009, 04:16 AM said:
I performed a western blot with anti-FLAG antibody (Cell Signaling) at a dilution of 1:200 in TBS (0.1%T-20). I expect to find a single band specific to the FLAG epitope tagged to my protein of interest but I ended up getting multiple bands. To make matter worse, I don't see any signal around the expected molecular weight of the my protein (deduced just from amino acid sequence).
So I have a couple of questions:
1. Will using a more diluted anti-FLAG Ab remove those unspecific bands? ( See the picture of the blot)
2. I used a 1:2000 dilution of the secondary antibody? Is that causing unspecific bands?
3. I expect my protein to be running around 75 KDa which is deduced just from amino acid sequence. So is there any way to know if it has a mol wt greater than 75 KDa. Thinking abt PTMs. Btw it is a transcription factor.
4. I used a untagged strain as a negative control (on the far right of the film besides the marker) and it shows faint bands adjacent to those multiple bands. Remember it is way under loaded.
5. Does anyone know of FLAG antibody binding non-specifically? I read somewhere in this forum about that problem.
Thank you. I would be glad to get any kind of suggestions.
I also used the anti-FLAG Ab form Cell Signal (there, it is differently named); we used a dilution of 1:1000, also for the secondaary Ab, all worked fine; you may also reduce the time of exposition
Julie123 on Apr 15 2010, 06:26 AM said:
laurequillo on Dec 7 2009, 09:48 AM said:
I can tell you how I do it. I use it at 1:5000 overnight, and then the secondary 1:10000 1.5-4 hours, and it gives me really nice blots. It is true that if the solution you use is new, sometimes you get background bands, but not big deal.
I saw cross reactivity with gfp and flag, but using high amount of proteins.
I am having the same problem with nonspecificity of anti-flag however ones from Sigma.Ihave tried monoclonal (get no signal) and polyclonal i see nonspecific band even in cells that don't have the flag vector.I have tried different dilutions of antibody and different dilutions of cell lysates still i get nonspecific signal.which cells are you using to prepare your lysates for western?
293(T), Hela, U2OS, C2C12... normally we dont have problem with the Flag antibody, it is quite good. The more you use an already diluted antibody the less background you will find. Anyway, even if you see background, how is the expression of your flag tagged proteins? how is the transfection efficiency?
