How to increase IF signal in acetone/methanol fixed cells? - (Sep/08/2009 )
I have 50:50 acetone/methanol fixed cells that look great by phase contrast but can't seem to get any fluorescence signal from them. They are blocked in 10% serum with .2% Tween20 and reacted overnight in a fairly high concentration of primary antibody (4ug/ml) in 1.5 % BSA. I am getting weak signal in the "no primary" controls but this is the same in samples exposed to the primary.
The results are the same from two different antibodies that work fine on Westernblots from the same cells and both antibodies are approved by the manufacturer (SCBT) for IF use. My target protein is a membrane-bound receptor so it should be present and is strongly positive by western with the same antibody.
Any ideas would be appreciated.
Try a different fixative - the acetone will dissolve the cell membrane, possibly releasing the receptors. I would try 2-4% paraformaldehyde.
Some epitopes just don't stand up well to methanol/acetone fixation.
Try 2-4% paraformaldehyde (15 minutes @ room temp), wash, and then permabilize. 0.5% triton-x (5-15 minutes @ r.t.) does a great job with permabilization
Well I had tried paraformaldehyde before but looking at my notes revealed that I had left out a permeablization step. 15 minutes in 0.5 % triton x100 did solve this problem and we are now getting good reactivity, at least with this polyclonal antibody. Picture attached.
Thanks,
AN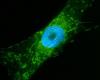
Nice picture!
Wow...mine's all blurry and even hard to see cell shape. Did you use confocal microscope?
