Help with unwanted fluorescent lines in sections - (Jun/14/2009 )
Hello. I'm new here and in need of some desperate help. I am using 4% PFA fixed mouse brain tissue. I use either mouse or rabbit antibody, and a Cy2 fluorescent mouse or rabbit antibody (donkey). I also use normal donkey serum for blocking. Many of my sections have intense, what looks like worms/lines all over the sections. It can't be the vasculature I am staining with the primary as well. Attached is an example of one of my slides with the ugly lines all over it. Any help would be greatly appreciated thanks.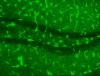
i dont get it - do you mean the bright lines all over tissue which look like positive staining (if a little over intense - dial down the miscroscope a bit)
you sure it isnt positive staining?
d
mmmmmm.......It does look quite positive that staining, like Dominic says. What is the magnification on the image?
And you used a mouse antibody on mouse brain? did you use something like a mouse-on mouse kit to reduce cross-reactivity or anything?
hi. the magnification there is 20x. i did not use the mouse on mouse kit, but i heard from others that should definitely be done prior to my immuno procedure. I sometimes see this (although to a much lesser extent) when using a rabbit primary on mouse tissue.
For sure the mouse on mouse kit should help reduce some of the background and things. I"m not a neurobiologist so I can't comment on what is staining there.
Did you do a secondary antibody control slide? No primary just the secondary to see if that reacts with anything?
here's the really weird part. i did do a control, no primary just secondary donkey anti-mouse and i did not see those lines, but got beautiful staining as if the primary was present. With no primary, there shouldn't be any staining, correct?
gaston on Jun 16 2009, 11:06 PM said:
Usually you shouldn't get any meaningful staining from secondary controls (non would be beautiful). I think your big problem is using the mouse antibodies, because your tissue is mouse then its going to be hard to tell what yr antibodies are really staining. It sounds like your primary is picking up something, Is it a well characterised antibody- as in lots of references on the data sheet you can check their staining against?
If you have another species antibody you can use- say Rabbit polyclonal what does the staining look like for that.
You are correct, there shouldn't be any staining if there was no primary (in an ideal world...). However, the presence of staining that was not found in your secondary only control indicates only one thing: that you have some staining from your primary. Do you know if the antibody detects only one band on a western blot? If it detects more than one, you could be getting non-specific primary staining.
Thank you for all your suggestions. Other ppl have suggested I use a mouse on mouse product to control for this. They usually get it from DAKO. Does anyone know the Cat# so I get it right the first time.
Just an aside question for anyone wishes to answer. Can a different secondary antibody play a big role in staining? For example, I usually use Cy2, but the staining is ok, not awesome, sometimes good, other times not. So maybe it's my primary or something wrong with my slices (ie. not perfused properly maybe???). Anyhow, today I tried for the first time Alexa 488, and lo and behold there were lots of immunoreactivity that I didn't see with Cy2.
Essentially they are the same anti rabbit or anti mouse, no?

I have also seen some differences with different secondary antibodies, sometimes it can be contamination or fluff in the tube (if its old). maybe you can try a quick centrifuge clean things up. Alexa fluor Ab are usually very good, you didn't add too much did you? usually I use at 1 in 800.
My labs have used the mouse on mouse kit from vector labs, they're very good.
https://www.vectorlabs.com/catalog.aspx
Anyway, I hope it helps,
Lost ![]()
