Cloning - Miniprep plasmid contaminated by gDNA? - (Sep/26/2015 )
I am having a weird problem with my minipreps. I sucessfully cloned my GOI into pQCIX-puro by infusion and T4 ligation. I picked ten colonies total from both preparations and culturered them overnight. Qiagen Miniprep went well and the yield and quality of the DNA was good. I sent out the DNA for seqeuncing and they all failed to prime with the 5' primer and had homopolymeric region repeats when using a primer that binds to the middle of my GOI.
I ran about 100-200ng of the plasmid on a gel (image 2006.jpg) and there are two bands: a thick band near the top of the wells that I think it's gDNA and a very faint band below it that could be my plasmid. Its hard to tell the size of it but it seems to be within the ladder range. My plasmid should be around 8.3kbp and I am using a 10kbp ladder. I also did a normal PCR with the the primer that binds to the middle of my GOI and the 3' primer that I used to PCR my GOI for cloning (image 1005.jpg). The product should be about 362bp, which is what I got.
Based on these results I think that the cloning went well but I somehow messed up the minipreps. I didnt do anything different from the protocol. The lysis step went slightly over 5mins (max ~30-60sec over) but I dont think this could have been the problem.
Does anyone have any suggetions or advice?

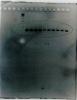
Hmmm, that could well be the problem - the timing of the lysis is critical, too long and the gDNA is nicked by the base in the extraction (usually NaOH) and will come out with the plasmid DNA, too short and there isn't enough time for the plasmid to unwind and the proteins to denature.
Stick to the time limit on the lysis and it should work fine.
