non-specific staining using with Immunos - (Aug/10/2012 )
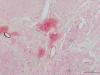
I have an antibody that I have had to troubleshoot with so much it's making me bananas. The staining itself is weak, and tends to get non-specific staining that masks true expression. It's a poly-clonal antibody, and I'm using it on FFPE sections 8µm thick using vector reagents. ALKP enzyme kits, and vector Red as my staining. I've upped the antibody to 1:25, do antigen unmasking with citrate buffer, quench endogenous ALKP with 0.2N HCl, and incubate 4˚C overnight with the primary antibody. I let the vector red incubate for 25-30 minutes, which is longer than I've ever needed to with other antibodies. I still get pink rings around my sections, or pink smears over my sections (2 jpegs below).
I'm doing something wrong, but I don't know what.
Reduce the time I incubate my primary antibody? Reduce the amount of time I leave the vector red on? something I haven't thought of?

Increasing the concentration of the antibody makes it more likely that you will see non-specific staining. Have you tried titrating the antibodies? How are you blocking?
Hi Bob1,
The blocking I've done is with 0.2 N HCl and normal serum, I haven't used powdered milk, BSA, etc.. do you have a method you prefer?
It may well be that the HCl is damaging the epitope of the protein in some manner, you could use a weaker acid, such as acetic acid for the quenching. Apparently formaldehyde fixation removes the majority of alkp activity normally. Have you tried something like an HRP based kit, which may get around the problem.
